Abstract
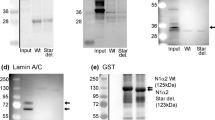
Dystrophin is a high molecular weight protein present at low abundance in skeletal, cardiac and smooth muscle and in trace amounts in brain. In skeletal muscle, dystrophin is uniformly distributed along the inner surface of the plasma membrane. Biochemical fractionation studies have shown that all detectable skeletal muscle dystrophin is tightly associated with a complex of wheat germ agglutinin (WGA)-binding and concanavalin A (Con A) binding sarcolemmal glycoproteins. Absence of dystrophin is the primary biochemical defect in patients with Duchenne muscular dystrophy and leads to segmental necrosis of their skeletal myofibers. Although present in similar amounts in normal cardiac and skeletal muscle, the absence of dystrophin from cardiac muscle has less severe effects on the survival of cardiac cells. We have therefore examined whether there are differences in the properties of cardiac and skeletal dystrophin. We report that in contrast to skeletal muscle, cardiac dystrophin is distributed between distinct pools: a soluble cytoplasmic pool, a membrane-bound pool not associated with WGA-binding glycoproteins and a membrane-bound pool associated with WGA-binding glycoproteins. Cardiac dystrophin was not associated with any Con A binding glycoproteins. Immunohistochemical localization studies in isolated ventricular myocytes reveal a distinct punctate staining pattern for dystrophin, approximating to the level of the transverse tubule/Z-line and contrasting with the uniform sarcolemmal staining reported for skeletal muscle fibers. The distinct properties of cardiac dystrophin suggest unique roles for this protein in cardiac versus skeletal muscle function.
Similar content being viewed by others
Abbreviations
- Dys:
-
Dystrophin
- T-tubule:
-
Transverse tubule
- SDS-PAGE:
-
Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis
- WGA:
-
Wheat Germ Agglutinin
- Con A:
-
Concanavalin A
- DHP:
-
Dihydropyridine receptor
- FITC:
-
Fluorescein Isothiocyanate Conjugate
- NAG:
-
N-Acetyl-D-Glucosamine
- NP-40:
-
NONIDET P-40
- PBS:
-
Phosphate-Buffered Saline
- TBST:
-
Tris Buffered Saline-Tween
References
Hoffman EP, Brown RH, Kunkel LM: Dystrophin: the protein product of DMD locus. Cell 51: 919–928, 1987
Hoffman EP, Hudecki MS, Rosenberg PA, Pollina CM, Kunkel LM: Cell and fibre-type distribution of dystrophin. Neuron 1: 411–420, 1988
Bennett V: Spectrin based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol Rev 70: 1029–1065, 1990
Watkins SC, Hoffman EP, Slayter HS, Kunkel LM: Immunoelectron microscopic localization of dystrophin in myofibres. Nature 333: 863–866, 1988
Carpenter SG, Karpati G, Zubrzycka-Gaarn E, Bulman DE, Ray PN, Worton RG: Dystrophin is localized to the plasma membrane of human skeletal muscle fibres by electron-microscopic cytochemical study. Muscle & Nerve 13: 376–385, 1990
Arahata K et al.: Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature 333: 861–863, 1988
Zubrzycka-Gaarn EE et al.: The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature 333: 466–469, 1988
Bonilla E et al.: Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell 54: 447–452, 1988
Brown RH, Hoffman EP: Molecular biology of Duchenne muscular dystrophy. Trends Neurosci 11: 480–485, 1988
Menke A, Jockusch H: Decreased osmotic stability of dystrophinless muscle cells frommdx mouse. Nature 349: 69–71, 1991
Ohlendieck K, Campbell KP: Dystrophin constitutes five percent membrane cytoskeleton in skeletal muscle. FEBS Lett 115: 1685–1694, 1991
Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP: Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature 345: 315–319, 1990
Engel AG: Myology ed. Engel AG, Banker BQ 1185 (McGraw Hill Book Co, New York, 1986)
Byers TJ, Kunkel LM, Watkins SC: The subcellular distribution of dystrophin in mouse skeletal, cardiac and smooth muscle. J Cell Biol 115: 411–422, 1991
Doucet J-P, Tuana BS: Identification of low molecular weight GTP-binding proteins and their sites of interaction in subcellular fractions from skeletal muscle. J Biol Chem 266: 17613–17619, 1991
Tuana BS, Murphy BJ, Yi Q: Subcellular distribution and isolation of the Ca2+ antagonist receptor associated with the voltage gated Ca2+ channel from rabbit heart. Mol Cell Biochem 80: 133–141, 1987
Désilets M, Baumgarten CM: K+, Na+ and Cl− activities in ventricular myocytes isolated from rabbit heart. Am J Physiol 251: C197, 1986
Sicinski P, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ: The molecular basis of muscular dystrophy in themdx mouse. Science 244: 1578–1580, 1989
Love DR, Hill DF, Dickson G, Spurr NK, Byth BC, Marsden RF, Walsh FS, Edwards YH, Davies KE: An autosomal transcript in skeletal muscle with homology to dystrophin. Nature 339: 55–59, 1989
Adams RJ, Schwartz A: Comparative mechanisms for contraction of cardiac and skeletal muscle. Chest 78: 1235–1395, 1980
Bers DM: Excitation-contraction coupling and Cardiac Contractile Force (Kluwer Academic Publishers, 1991) pp 17–32, Page SG, The Physiological Basis of Starling's Law of the Heart (ed Ciba Foundation Symposium) 13, (Associated Scientific Publishers, Amsterdam, 1974)
Ervasti JM, Kahl SD, Campbell KP: Purification of dystrophin from skeletal muscle. J Biol Chem 266: 9161–9165, 1991
Ervasti JM, Campbell KP: Membrane organization of the dystrophin-glycoprotein complex. Cell 66: 1121–1131, 1991
Yoshida M, Ozawa E: Glycoprotein complex anchoring dystrophin to sarcolemma. J Biochem 108: 748–752, 1990
Ibraghimov-Beskrovnaya O et al.: Primary structure of dystrophin associated glycoproteins linking dystrophin to the extracellular matrix. Nature 355: 696–702, 1992
Frank JS, Mottino G, Reid D, Molday RS, Philipson KD: Distribution of the Na++Ca2+ exchanger in mammalian cardiac myocytes: An immunofluorescence and immunocollodial gold-labeling study. J Cell Biol 251: 1451–1455, 1991
Fong P, Turner PR, Denetclaw WF, Steinhardt RA: Increased activity of Ca2+ leak channels in myotubes of Duchenne human andmdx mouse origin. Science 250: 673–676, 1990
Franco A, Lansman JB: Ca2+ entry through stretch inactivated ion channels inmdx myotubes. Nature 344: 670–673, 1990
Feener CA, Koenig M, Kunkel LM: Alternative splicing of human dystrophin mRNA generates isoforms at the carboxy terminus. Nature 338: 509–511, 1989
Bies RD, Friedman D, Roberts R, Perryman MB, Caskey CJ: Expression and localization of dystrophin in human cardiac purkinje fibres. Circulation 86: 147–153, 1992
Perloff JK: Cardiac rhythm and conduction in Duchenne's muscular dystrophy. J Am Coll Cardiol 3: 1263–1268, 1988
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Peri, V., Ajdukovic, B., Holland, P. et al. Dystrophin predominantly localizes to the transverse tubule/Z-line regions of single ventricular myocytes and exhibits distinct associations with the membrane. Mol Cell Biochem 130, 57–65 (1994). https://doi.org/10.1007/BF01084268
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01084268