Abstract
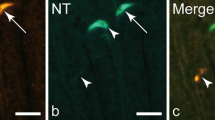
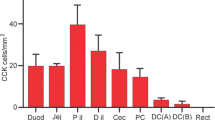
Rat intestinal 15 kDa protein (I-15P) is highly homologous to porcine gastrotropin. We studied the occurrence, distribution and subcellular localization of I-15P in the entire rat body, using the immunocytochemistry to localize protein andin situ hybridization to localize mRNA. Both techniques demonstrated the expression of I-15P in the enterocytes of ileum, luteal cells of ovary and a subpopulation of steroid-endocrine cells of adrenal gland. Immuno-electron microscopy further demonstrated that I-15P is localized in both the cytoplasmic and nuclear matrix regions of these cells. The present results suggest roles of I-15P not only in the transport of bile salts but also in the metabolisms of certain steroid hormones.
Similar content being viewed by others
References
Glatz FC, Van der Vusse GJ: Cellular fatty acid-binding proteins: current concepts and future directions. Mol Cell Biochem 98: 237–251, 1990
Wider MD, Vinik AI, Heldsinger A: Isolation and partial characterization of an enterooxyntin from porcine ileum. Endocrinology 115: 1484–1491, 1984
Wider MD, Duhaime PQM, Weisman RL: Chemical characterization of circulating procine ileal polypeptide in plasma from normal adult pigs. Endocrinology 118: 1456–1551, 1986
Tsunoda Y, Wider MD: Porcine ileal polypeptide causes an increase in cytoplasmic Ca2+ in both parietal and chief cells resulting in acid and pepsinogen secretion. Biochim Biophys Acta 905: 118–124, 1987
Walz DA, Wider MD, Snow JW, Desiderio DM: The complete amino acid sequence of porcine gastrotropin, an ileal protein which stimulates gastric acid and pepsinogen secretion. J Biol Chem 263: 14189–14195, 1988
Gantz I, Northwehr SF, Lucey M, Sacchettini JC, DelValle J, Banaszak LJ, Naud M, Gordon JI, Yamada T: Gastrotropin: Not an enterooxyntin but a member of a family of cytoplasmic hydrophobic ligand binding proteins. J Biol Chem 264: 20248–20254, 1989
Sacchettini JC, Hauft SM, Van Camp SL, Cistola DP, Gordon JI: Developmental and structural studies of an intracellular lipid binding protein expressed in the ileal epithelium. J Biol Chem 265: 19199–19207, 1990
Borgstrom AM, Wider M, Marks W, Loyd R, Herman G, Vinik A: Immunohistochemical localization of a specific ileal peptide in the pig. Histochemistry 86: 101–105, 1986
Gordon JI, Elshourbagy N, Lowe JB, Liao WS, Alpers DH, Taylor JM. Tissue specific expression and developmental regulation of two genes coding for rat fatty acid binding proteins. J Biol Chem 260: 1995–1998, 1985
Bass GG, Manning JA: Tissue expression of three structurally different fatty acid binding proteins from rat heart muscle, liver, and intestine. Biochem Biophys Res Commun 137: 929–935, 1986
Shields H M, Bates ML, Bass NM, Best CJ, Alpers DH, Ockner RK: Light microscopic immunocytochemical localization of hepatic and intestinal types of fatty acid-binding proteins in rat small intestine. J Lipid Res 27: 549–557, 1986
Kanda T, Ono T, Matsubara Y, Muto T: Possible role of rat fatty-acid-binding proteins in the intestine as carriers of phenol and phthalate derivatives. Biochem Biophys Res Commun 168: 1053–1058, 1990
Kanda T, Odani S, Tomoi M, Matsubara Y, Ono T: Primary structure of a 15 kDa protein from rat intestinal epithelium: Sequence homology to fatty acid-binding proteins. Eur J Biochem 197: 759–768, 1991
Iseki S, Kondo H, Hitomi M, Ono T: Immunocytochemical localization of hepatic fatty acid binding protein in the liver of fed and fasted rats. Histochemistry 89: 317–322, 1988
Iseki S, Hitomi M, Ono T, Kondo H: Immunocytochemical localization of hepatic fatty acid binding protein in the rat intestine: effect of fasting. Anat Rec 223: 283–291, 1989
Iseki S, Kondo H: Light microscopic localization of hepatic fatty acid binding protein mRNA in jejunal epithelia of rats usingin situ hybridization, immunohistochemical, and autoradiographic techiques. J Histochem Cytochem 38: 111–115, 1990
Iseki S, Kondo H, Hitomi M, Ono T: Localization of liver fatty acid-binding protein and its mRNA in the liver and jejunum of rats: an immunohistochemical andin situ hybridization study. Mol Cell Biochem 98: 27–33, 1990
Amano O, Kanda T, Ono T, Iseki I: Immunocytochemical localization of rat intestinal 15 kDa protein, a member of cytoplasmic fatty acid-binding proteins. Anat Rec, 234: 215–222, 1992
Lin MC, Gong Y, Geoghegan KF, Wilson FA: Characterization of a novel 14 kDa bile acid-binding protein from the rat ileal cytosol. Biochim Biophys Acta 1078: 329–335, 1991
Vodenlich Jr AD, Gong Y, Geoghegan, KF, Lin MC, Lanzetti AJ, Wilson FA: Identification of the 14 kDa bile acid transport protein of rat ileal cytosol as gastrotropin. Biochem Biophys Res Commun 177: 1147–1154, 1991
Bordewick U, Heese M, Börchers T, Robenek H, Spener F: Compartmentation of hepatic fatty-acid-binding protein in liver cells and its effect on microsomal phosphatidic acid biosynthesis. Biol Chem Hoppe-Seyler 370: 229–238, 1989
Börchers T, Unterberg C, Rudel H, Robenek H, Spener F: Subcellular distribution of cardiac fatty acid-binding protein in bovine heart muscle and quantitation with an enzyme-linked immunosorbent assay. Biochim Biophys Acta 1002: 54–61, 1989
Watanabe M, Ono T, Kondo H: Immunohistochemical studies on the localisation and ontogeny of heart fatty acid binding protein in the rat. J Anat 174: 81–95, 1991
Capron F, Coltoff-Schiller B, Johnson AB, Fleischner GM, Goldfischer S: Immunocytochemical localization of hepatic ligandin and Z protein utilizing frozen sections for light and electron microscopy. J Histochem Cytochem 27: 961–966, 1979
Crisman TS, Claffey KP, Saouaf R, Hanspal J, Brecher P: Measurement of rat heart fatty acid-binding protein by ELISA. Tissue distribution, developmental changes and subcellular distribution. J Mol Cell Cardiol 19: 423–431, 1987
Guraya SS: Comparative studies on the histochemical features of ovarian components in the rat and golden hamster, with special reference to steroid hormone synthesis. Acta Anat 82: 284–304, 1972
McDonald DM, Seiki K, Prizant M, Goldfien A: Ovarian secretion of progesterone in relation to the Golgi apparatus in lutein cells during the estrous cycle of the rat. Endocrinology 85: 236–243, 1969
Strauss III JF, Schuler LA, Rosenblum MF, Tanaka T: Cholesterol metabolism by ovarian tissue. Adv Lipid Res 18: 99–157, 1981
Jansen H, Hulsman WC: Heparin-releasable (liver) lipase(s) may play a role in the uptake of cholesterol by steroid-secreting tissues. Trends Biochem Sci 5: 265–268, 1980
Jansen H, De Greef WJ, Uilenbroek JTJ: Localization of liver-type lipase in rat ovaries and its activity during the estrous cycle and lactation. Mol Cell Endocrinol 42: 253–258, 1985
Hixenbaugh EA, Paavola LG: Heterogeneity among ovarian blood vessels: endogenous hepatic lipase is concentrated in blood vessels of rat corpora lutea. Anat Rec 230: 291–306, 1991
Long JA: Zonation of the mammalian adrenal cortex. In: Hand-book of Physiology, Sec 7, Vol VI, American Physiology Society, Washington, DC, 1975, pp 13–24
Bornstein SR, Ehrhart-Bornstein M, Usadel H, Böckmann M, Scherbaum W: Morphological evidence for a close interaction of chromaffin cells with cortical cells within the adrenal gland. Cell Tissue Res 265: 1–9, 1991
Veerkamp JH, Paulussen RJA, Peeters RA, Maatman RGHJ, Van Moerkerk HTB, Van Kuppevelt THMSM: Detection, tissue distribution and (sub)cellular localization of fatty acid-binding protein types. Mol Cell Biochem 98: 11–18, 1990
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Iseki, S., Amano, O., Kanda, T. et al. Expression and localization of intestinal 15 kDa protein in the rat. Mol Cell Biochem 123, 113–120 (1993). https://doi.org/10.1007/BF01076482
Issue Date:
DOI: https://doi.org/10.1007/BF01076482