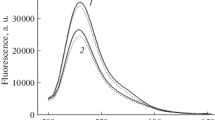
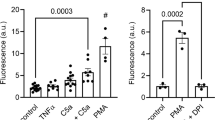
Abstract
A chemotactic peptide, N-formyl-methionyl-leucyl-phenylalanine (fMLP), induced an acidification of cytosol by about 0.05 pH units in 30 sec followed by an alkalinization in human neutrophils. The quantitative contribution of acid production to the acidification was studied. The superoxide (O2 −) production stimulated by fMLP was not involved in the acidification because the production of acids in neutrophils from patients with chronic granulomatous disease who do not produce O2 −, was the same as that in normal neutrophils. The intracellular acidification was completely inhibited by deoxyglucose, suggesting that energy metabolism enhanced upon stimulation by fMLP might be the main source of the acidification. Although enhancement of the lactate formation by fMLP was 0.8 nmol/106 cells, which could lower intracellular pH by 0.08 pH units, the lactate production could not explain the initial acidification because the production of lactate started at 1 min after the stimulation while the intracellular acidification began immediately after the stimulation. Mitochondrial respiratory inhibitors such as KCN and rotenone had no effects on the fMLP-induced intracellular acidification. The fMLP-induced production of CO2 in 30 sec through the hexose monophosphate shunt was only 2.6 pmol/106 cells, which was calculated to decrease intracellular pH by only 0.0014. Thus, changes of energy metabolism induced by fMLP does not explain the acidification.
Similar content being viewed by others
Abbreviations
- fMLP:
-
N-formyl-methionyl-leucyl-phenylalanine
- BCECF-AM:
-
2′,7′-bis(carboxyethyl)carboxyfluorescein acetoxymethyl ester
- PMA:
-
phorbol 12-myristate 13-acetate
- CGD:
-
chronic granulomatous disease
- HMP:
-
hexose monophosphate
- pHi:
-
intracellular pH
References
Frelin C, Vigne P, Ladoux A, Lazdunski M: The regulation of the intracellular pH in cells from vertebrates. Eur J Biochem 174: 3–14, 1988
Madshus IH: Regulation of intracellular pH in eukaryotic cells. Biochem J 250: 1–8, 1988
Roos A, Boron WF: Intracellular pH. Physiol Rev 61: 296–434, 1981
Taggart MJ, Wray S: Occurrence of intracellular pH transients during spontaneous contractions in rat uterine smooth muscle. J Physiol 472: 23–31, 1993
Fleming I, Hecker M, Busse R: Intracellular alkalinization induced by bradykinin sustains activation of the constitutive nitric oxide synthase in endothelial cells. Circulation Res 74: 1220–1226, 1994
Hartley Z, Dubinsky JM: Changes in intracellular pH associated with glutamate excitotoxicity. J Neuroscience 13: 4690–4699, 1993
Klebanoff SJ, Clark RA: The Neutrophil. North-Holland Publishing, Amsterdam, 1978
Grinstein S, Furuya W: Cytoplasmic pH regulation in phorbol esteractivated human neutrophils. Am J Physiol 250: C55-C65, 1986
Simchowitz L: Intracellular pH modulates the generation of superoxide radicals by human neutrophils. J Clin Invest 76: 1079–1089, 1985
Osaki M, Sumimoto H, Takeshige K, Cragoe EJJ, Hori Y, Minakami S: Na+/H+ exchange modulates the production of leukotrien B4 by human neutrophils. Biochem J 257: 751–758, 1989
Satoh M, Nanri H, Takeshige K, Minakami S: Pertussis toxin inhibits intraceliular pH changes in human neutrophils stimulated by N-formyl-methionyl-leucyl-phenylalanine. Biochem Biophys Res Commun 131: 64–69, 1985
Nanda A, Gukovskaya A, Tseng J, Grinstein S: Activation of vacuolartype proton pumps by protein kinase C. J Biol Chem 267: 22740–22746, 1992
Kapus A, Susztak K, Ligeti E: Regulation of the electrogenic H+ channel in the plasma membrane of neutrophils. Biochem J 292: 445–450, 1993
Nanda A, Grinstein S, Curnette JT: Abnormal activation of H+ conductance in NADPH oxidase-defective neutrophils. Proc Natl Acad Sci USA 90: 760–764, 1993
Sumimoto H, Satoh M, Takeshige K, Cragoe EJJ, Minakami S: Cytoplasmic pH change induced leukotrien B4 in human neutrophils. Biochim Biophys Acta 970: 31–38, 1988
Bernardo J, Brennan L, Brink HF, Ortiz MF, Newburga PE, Simmons ER: Chemotactic peptide-induced cytoplasmic pH changes in incubated human monocytes. J Leukocyte Biol 53: 673–678, 1993
Sullivan R, Griffin JD, Wright J, Melnick DA, Leavitt JL, Fredette JP, Horne JHJ, Lyman CA, Lazzari KG, Simons ER: Effects of recombinant human granulocyte-macrophage colony-stimulating factor on intracellular pH in mature granulocytes. Blood 72: 1665–1673, 1988
Boyüm A: Isolation of mononuclear cells and granutocytes from human blood. Scand J Clin Lab Invest 21 (suppl. 97) 77–391, 1968
Thomas JA, Buchsbaum RN, Zimniak A, Racker E: Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generatedin situ. Biochemistry 18: 2210–2218, 1979
Lehrer RI: Effects of colchicine and chloramphenicol on the oxidative metabolism and phagocytic activity of human neutrophils. J Infect Disease 127: 40–48, 1973
Minakami S: Studies on leucocyte metabolism. J Biochem 63: 83–88, 1968
Grinstein S, Furuya W, Biggar WD: Cytoplasmic pH regulation in normal and abnormal neutrophils. J Biol Chem 261: 512–514, 1986
Volpi M, Naccache PH, Molski TFP, Shefyck J, Huang CK, Marsh ML, Munoz J, Becker EL, Sha'afi Rl: Pertussis toxin inhibits fMet-Leu-Phe but not phorbol ester stimulated changes in rabbit neutrophils: Role of G proteins in excitation response coupling. Proc Natl Acad Sci USA 82: 2708–2712, 1985
Borregard N, Herlin T: Energy metabolism of human neutrophils during phagocytosis. J Clin Invest 70: 550–557, 1982
Newby AC, Holmquirt CA: Adenosine production inside rat polymorphonuclear leucocytes. Biochem J 200: 399–403, 1981
Weisman SJ, Punzo A, Ford C, Sha'afi R: Intracellular pH change during neutrophil activation. J Leukocyte Biol 41: 25–32, 1987
Naccache PH, Therrien S, Caon AC, Liao N, Gilbert C, McColl SR: Chemoattractant-induced cytoplasmic pH changes and cytoskeletal reorganization in human neutrophils. J Immunol 142: 2438–2444, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Satoh, M., Asagami, H., Kang, D. et al. Quantitative contribution of the acid production to the intracellular acidification in human neutrophils stimulated by N-formyl-methionyl-leucyl-phenylalanine. Mol Cell Biochem 152, 159–165 (1995). https://doi.org/10.1007/BF01076078
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01076078