Abstract
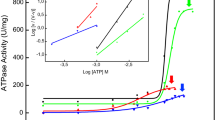
Alkaline phosphatase activity was released up to 100% from the membrane by incubating the rat osseous plate membrane-bound enzyme with phosphatidylinositol-specific phospholipase C. The molecular weight of the released enzyme was 145,000 on Sephacryl S-300 gel filtration and 66,000 on PAGE-SDS, suggesting a dimeric structure. Solubilization of the membrane-bound enzyme with phospholipase C did not destroy its ability to hydrolyse PNPP, ATP and pyrophosphate. The hydrolysis of ATP and PNPP by phosphatidylinositol-specific phospholipase C-released enzyme exhibited ‘Michaelian’ kinetics with K0.5=70 and 979 μM, respectively. For pyrophosphate, K0.5 was 128 μM and site-site interactions were observed (n=1.4). Magnesium ions were stimulatory (K0.5=1.5 mM) and zinc ions were a powerful noncompetitive inhibitor (Ki=6.2 μM) of phosphatidylinositol-specific phospholipase C-released enzyme.
Phosphatidylinositol-specific phospholipase C-released alkaline phosphatase was relatively stable at 40°C. However, with increasing temperature from 40–60°C, the enzyme was inactivated rapidly following first order kinetics and thermal inactivation constants varied from 5.08×10−4 min−1 to 0.684 min−1.
Treatment of phosphatydilinositol-specific phospholipase C-released alkaline phosphatase with Chellex 100 depleted to 5% its original PNPPase activity. Magnesium (K0.5=29.5 μM), manganese (K0.5=5 μM) and cobalt ions (K0.5=10.1 μM) restored the activity of Chelex-treated enzyme, demonstrating its metalloenzyme nature. The stimulation of Chelex-treated enzyme by calcium ions (K0.5=653 μM) was less effective (only 26%) and occurred with site-site interactions (n=0.7). Zinc ions had no stimulatory effects.
The possibility that the soluble form of the enzyme, detected during endochondral ossification, would arise by the hydrolysis of the P1-anchored form of osseous plate alkaline phosphatase is discussed.
Similar content being viewed by others
References
Low MG: Biochemistry of the glycosyl-phosphatydilinositol membrane protein anchors. Biochem J 244: 1–13, 1987
Low MG, Ferguson MAJ, Futerman AH, Silman l: Covalently attached phosphatydilinositol as a hydrophobic anchor for membrane proteins. Trends Biochem Sci 11: 212–215, 1986
Fergusson MAJ: Glycosyl-phosphatidylinositol membrane anchors: the tale of a tail. Biochem Soc Transactions 20: 243–256, 1992
Ferguson MAJ, Willians AF: Cell surface anchoring of proteins via glycosyl phosphatydilinositol structures. Ann Rev Biochem 57: 285–320, 1988
Low MG, Saltiel AR: Structural and functional roles of glycosyl phosphatydilinositol in membranes. Science 239: 268–275, 1988
Saltiel AR, Ravetch J, Aderem AA: Functional consequences of lipid-mediated protein-memrane interactions. Biochem Pharmacol 42: 1–11, 1991
Slein MW, Logan GF: Characterization of the phospholipases of Bacillus cereus and their effects on erythrocytes, bone and kidney cells. J Bacteriol 90: 69–81, 1965
Ikezawa H, Yamanegi M, Taguchi R, Miyashita T, Ohyabu T: Studies on phosphatydilinositol phosphodiesterase (phospholipase C type) of Bacillus cereus. Biochim Biophys Acta 450: 154–164, 1976
Low MG, Finean JB: Release of alkaline phosphatase from membranes by a phosphatydilinositol-specific phospholipase C. Biochem J 167: 281–284, 1977
Ali SY, Sajdera SW, Anderson HC: Isolation and characterization of calcifying vesicles from epiphyseal cartilage. Proc Natl Acad Sci USA 67: 1513–1520, 1970
Matsuzawa T, Anderson HC: Phosphatases of epiphyseal cartilage studied by electron microscopic cytochemical methods. J Hystochem Cytochem 19: 801–808, 1971
Majeska RJ, Wuthier RE: Studies on matrix vesicles isolated from chick epiphyseal cartilage. Association of pyrophosphatase and ATPase activities with alkaline phosphatase. Biochim Biophys Acta 391: 51–60, 1975
Felix R, Fleisch H: Pyrophosphatase and ATPase of isolated cartilage matrix vesicles. Calcif Tissue Res 22: 1–7, 1976
Robison R: The possible significance of hexose phosphoric esters in ossification. Biochem J 17: 286–293, 1923
Register TC, McLean FM, Low MG, Wuthier RE: Roles of alkaline phosphatase and labile internal mineral in matrix vesicle-mediated calcification. Effect of selective release of membrane-bound alkaline phosphatase and treatment with isosmotic pH 6 buffer. J Biol Chem 261: 9354–9360, 1986
Cyboron GW, Wuthier RE: Purification and initial characterization of intrinsic membrane-bound alkaline phosphatase from chicken epiphyseal cartilage. J Biol Chem 256: 7262–7268, 1981
Stagni N, Vittur F, DeBernard B: Solubility properties of alkaline phosphatase from matrix vesicles. Biochim Biophys Acta 761: 246–251, 1983
Wuthier RE, Register TC: Role of alkaline phosphatase: a polyfunctional enzyme in mineralizing tissues. In: W.T. Butler (ed.). The Chemistry and Biology of Mineralized Tissue. EBSCO Media Inc. Birminghan, 1985, pp 113–124
Curti C, Pizauro JM, Rossinholi G, Vugman I, Mello de Oliveira JA, Leone FA: Isolation and kinetic properties of an alkaline phosphatase from rat bone matrix induced cartilage. Cell Mol Biol 32: 55–62, 1986
Say JC, Ciuffi K, Furriel RPM, Ciancaglini P, Leone FA: Alkaline phosphatase from rat osseous plates: purification and biochemical characterization of a soluble form. Biochim Biophys Acta 1074: 256–262, 1991
Davitz MA, Hereld D, Shak S, Krakow J, Englund PT, Nussenzweig V: A glycan-phosphatydilinositol-specific phospholipase D in human serum. Science 238: 81–84, 1987
Low MG, Prasad ARS: A phospholipase D specific for the phosphatidyl inositol anchor cell surface protein is abundant in plasma. Proc Natl Acad Sci USA 85: 980–984, 1988
Low MG, Zilversmith DB: Role of phosphatydilinositol in attachment of alkaline phosphatase to membranes. Biochemistry 19: 3913–3918, 1980
Ferguson MAJ, Homans SW, Dwek RA, Rademacher TW: Glycosylphosphatydilinositol moyety that anchor Trypanosoma brucei variant surface glycoprotein to the membrane. Science 239: 753–759, 1988
Cardoso de Almeida ML, Turner MJ, Stambuk BB, Schenkman N: Identification of an acid lipase in human serum which is capable of solubilizing glycophosphatydil-inositol-anchored proteins. Biochem Biophys Res Comm 150: 476–482, 1988
Huang KS, Li S, Fuhg WJC, Hulmes JD, Reik L, Pan YCE, Low MG: Purification and characterization of glycosylphosphatidylinositol specific phospholipase D. J Biol Chem 265: 17738–17745, 1990
Hoener MC, Brodbec KU: Phosphatidylinositol-glycan-specific phospholipase D is an amphiphilic glycoprotein that in serum is associated with high-density lipoproteins. Eur J Biochem 206: 747–757, 1992
Stinson RA, Hamilton BA: Human liver plasma membranes contain an enzyme activity that removes membrane anchor from alkaline phosphatase and converts it to a plasma-like form. Clin Biochem 27: 49–55, 1994
Heinonen JK, Lahti RJ: A new convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Analyt Biochem 113: 313–317, 1981
Davis BJ: Disc electrophoresis. Method and application to human serum proteins. Ann NY Acad Sci 121: 404–427, 1964
Blum H, Beir H, Gross HT: Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8: 93–99, 1987
Hartree EF: Determination of protein: a modification of the Lowry method that gives a linear photometric response. Analyt Biochem 48: 422–427, 1972
Read SM, Northcote DH: Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Analyt Biochem 116: 53–64, 1981
Weber K, Osborn M: The reliability of molecular weight determination by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem 244: 4406–4412, 1969
Ciancaglini P, Pizauro JM, Leone, FA: Polyoxyethylene 9-lauryl ether-solubilized alkaline phosphatase: synergistic stimulation by zinc and magnesium ions. Int J Biochem 24: 611–615, 1992
Leone FA, Pizauro JM, Ciancaglini P: Effect of pH on the modulation of rat osseous plate alkaline phosphatase by metal ions. Int J Biochem 24: 923–928, 1992
Lowe M, Strauss AW, Alpers R, Seetharam S, Alpers DH: Molecular cloning and expression of a cDNA encoding the membrane-associated rat intestinal alkaline phosphatase. Biochem Biophys Acta 1037: 170–177, 1990
Goldstein DJ, Rogers C, Harris H: A search for trace expression of placental-like alkaline phosphatase in non-malignant human tissues: demonstration of its occurrence in lung, cervix, testis and thymus. Clin Chim Acta 125: 63–75, 1982
Nakamura T, Nakamura K, Stinson RA: Release of alkaline phosphatase from human osteosarcoma cells by phosphatidylinositol phospholipase C: effect of tunicamycin. Arch Biochem Biophys 265: 190–196, 1988
Collin P, Nefussi JR, Wetterwald A, Nicolas V, Boy-Lefrevre ML, Fleish H, Forest N: Expression of collagen, osteocalcin, and bone alkaline phosphatase in a mineralizing rat osteoblastic cell culture. Calcif Tissue Int 50, 175–183, 1992
Kiledjian M, Kadesch T: Post-transcriptional regulation of the human liver/bone/kidney alkaline phosphatase gene. J Biol Chem 266: 4207–4213, 1991
Rezende AA, Pizauro JM, Ciancaglini P, Leone FA: Phosphodiesterase activity is a novel property of alkaline phosphatase from osseous plate. Biochem J 301: 517–522, 1994
Ciancaglini P, Pizauro JM, Rezende AA, Rezende LA, Leone FA: Solubilization of membrane-bound matrix- induced alkaline phosphatase with polyoxyetylene 9-lauryl ether (Polidocanol): Purification and metalloenzyme properties. Int J Biochem 22: 385–392, 1990
Pizauro JM, Curti C, Ciancaglini P, Leone FA: Triton X-100 solubilized bone matrix-induced alkaline phosphatase. Comp Biochem Physiol 67B: 921–926, 1987
Pizauro JM, Cianeaglini P, Leone FA: Allosteric modulation by ATP. calcium and magnesium ions of rat osseous plate alkaline phosphatase. Biochim Biophys Acta 1202: 22–28, 1993
Ikezawa H, Taguchi R: Phosphatidylinositol-specific phospholipase C fromBacillus cereus andBacillus thuringiensis. Methods Enzymol 71: 731–741, 1981
Little C: Phospholipase C fromBacillus cereus. Methods Enzymol 71: 725–730, 1981
Takahashi T, Sugahara T, Ohsaka A: Phospholipase CClostridium pefringens. Methods Enzymol 71: 710–724, 1981
Curti C, -Pizauro JM, Ciancaglini P, Leone FA: Characteristics of some inhibitors of matrix-induced alkaline phosphatase. Cell Mol Biol 33: 625–635, 1987
Pizauro JM, Curti C, Ciancaglini P, Leone FA: Kinetic properties of Triton X-100 solubilized bone matrix-induced alkaline phosphatase. Cell Mol Biol 34: 553–562, 1988
Dixon M: The effect of pH on the affinities of enzymes for substrates and inhibitors. Biochem J 55: 161–170, 1953
McComb RB, Bowers GN, Posen S: Reaction Mechanisms. In: Alkaline phosphatase. Plenum Press, New York, 1979, pp 229–287
Nayadu PRV, Hercus B: Molecular hererogeneity of mouse duodenal alkaline phosphatase. Biochem J 141: 93–101, 1974
Bublitz R, Armesto J, Hoffmann-Blume E, Schulze M, Rhode H, Horn A, Aulwurm S, Hannappel E, Fischer W: Heterogeneity of glycocyl phospatidylinositol-anchored alkaline phosphatase of calf intestine. Eur J Biochem 217: 199–207, 1993
Deng JT, Hoylaerts MF, VanHoof VO, DeBroe ME: Differential release of human intestinal alkaline phosphatase in duodenal fluid and serum. Clin Chem 38: 2532–2538, 1992
Duval N, Krejci E, Grassi J, Cousson F, Massoulie J, Bon S: Molecular architecture of acetylcholinesterase colagen-tailed forms; construction of a glycolipid-tailed tetramer. EMBO J 11: 3255–3261, 1992
Helenius A, Simons K: Solubilization of membranes by detergents. Biochim Biophys Acta 415: 29–79, 1975
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pizauro, J.M., Ciancaglini, P. & Leone, F.A. Characterization of the phosphatidylinositol-specific phospholipase C-released form of rat osseous plate alkaline phosphatase and its possible significance on endochondral ossification. Mol Cell Biochem 152, 121–129 (1995). https://doi.org/10.1007/BF01076074
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01076074