Abstract
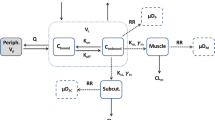
Pharmacokinetic data from 20-min constant rate infusions of the ACE inhibitor S-9780 1 mg to 16 subjects were studied for evidence of nonlinearity. A hierarchy of standard compartmental models and of nonlinear binding models was fitted to the data by least squares nonlinear regression and the most appropriate model was chosen on the basis of F-ratio tests, Schwarz criteria, and residual plots. A one-compartment model which included saturable tissue and plasma binding components allowed the best overall description of the data. Median parameter estimates from this model suggest that approximately 308 nmol of plasma binding sites and 572 nmol of tissue binding sites were present and that the total plasma concentration of S-9780 at 50% saturation of binding sites was 16.5 nmol L−1. The elimination half-life for free drug in plasma was only 30 min. This model describes the discrepancy previously noted between accumulation and apparent elimination half-lives for long-acting ACE inhibitors and offers a noninvasive method for assessment of tissue-bound ACE inhibitorin vivo.
Similar content being viewed by others
References
N. Hockings, A. A. Ajayi, and J. L. Reid. Age and the pharmacokinetics of angiotension converting enzyme inhibitors enalapril and enalaprilat.Br. J. Clin. Pharmacol. 21:341–348 (1986).
K. R. Lees and J. L. Reid. Effects of intravenous S-9780, an angiotensin-converting enzyme inhibitor, in normotensive subjects.J. Cardiovasc. Pharmacol. 10:129–135 (1987).
A. E. Till, H. J. Gomez. M. Hichens, J. A. Bolognese, W. R. McNabb, B. A. Brooks, F. Noormohamed, and A. F. Lant. Pharmacokinetics of repeated single oral doses of enalapril maleate (MK-421) in normal volunteers.Biopharm. Drug Dispos. 5:273–280 (1984).
P. J. McNamara, G. Levy, and M. Gibaldi. Effect of plasma protein and tissue binding on the time course of drug concentration in plasma.J. Pharmacokin. Biopharm. 7:195–206 (1979).
S. Øie, T. W. Guentert, and T. N. Tozer. Effect of saturable binding on the pharmacokinetics of drugs: A simulation.J. Pharm. Pharmacol. 32:471–477 (1980).
P. J. McNamara, J. T. Slattery, M. Gibaldi, and G. Levy. Accumulation kinetics of drugs with nonlinear plasma protein and tissue binding characteristics.J. Pharmacokin. Biopharm. 7:397–405 (1979).
R. J. Francis, A. N. Brown, L. Kler, T. Fasanella d'Amore, J. Nussberger, B. Waeber, and H. R. Brunner. Pharmacokinetics of the converting enzyme inhibitor cilazapril in normal volunteers and the relationship to enzyme inhibition: development of a mathematical model.J. Cardiovasc. Pharmacol. 9:32–38 (1987).
K. R. Lees, S. T. Green, and J. L. Reid. Influence of age on the pharmacokinetics and pharmacodynamics of perindopril.Clin. Pharmacol. Ther. 44:418–425 (1988).
D. J. Tocco, F. A. de Luna, A. E. W. Duncan, T. C. Vassil, and E. H. Ulm. The physiological disposition and metabolism of enalapril maleate in laboratory animals.Drug Metab. Dispos. 10:15–19 (1982).
S. G. Chiknas. A liquid chromatography-assisted assay for angiotensin converting enzyme (peptidyl dipeptidase) in serum.Clin. Chem. 25:1259–1262 (1979).
D. W. Cushman and H. S. Cheung. Spectrophotometric assay and properties of the angiotensin converting enzyme of rabbit lung.Biochem. Pharmacol. 20:1638–1648 (1971).
M. Ralston. Derivative-free nonlinear regression. In W. J. Dixon, M. B. Brown, L. Engelman, J. W. Frane, M. A. Hill, R. I. Jennrich, and J. D. Toporek (eds.)BMDP Statistical Software, California Press, Loa Angeles, CA, 1983, Chap. 14.2, pp. 305–314.
J. Neter and W. Wasserman, Inferences in regression analysis. InApplied Linear Statistical Models, Irwin, Homewood, IL, 1974, Chap. 7, pp. 87–92.
G. T. Schwarz. Estimating the dimension of a model.Ann. Statist. 6:461–464 (1978).
B. Efron and G. Gong. A leisurely look at the bookstrap, the jackknife and cross-validation.Am. Statist. 37:36–48 (1983).
J. R. Harrigan, D. M. Hughes, and P. A. Meredith. Characterising interspecies differences in ACE inhibition.Br. J. Clin. Pharmacol. 27:656P (1989).
J. W. Ryan. Assay of peptidase and protease enzymes in vivo.Biochem. Pharmacol. 32:2127–2137 (1983).
C. J. Lindsey, L. M. Bendhack, and A. C. M. Paiva. Effects of teprotide, captopril and enalaprilat on arterial wall kininase and angiotensin converting activity.J. Hypertension 5(Suppl. 2):S71-S76 (1987).
V. J. Dzau, J. Rosenthal, and J. D. Swales. Vascular renin-A consensus view.J. Hypertension 5 (Suppl. 2):S77-S78 (1987).
Author information
Authors and Affiliations
Additional information
The authors are grateful to IRIS, Neuilly sur Seine, France for supplies of S-9780 and for financial support.
Rights and permissions
About this article
Cite this article
Lees, K.R., Kelman, A.W., Reid, J.L. et al. Pharmacokinetics of an ACE inhibitor, S-9780, in man: Evidence of tissue binding. Journal of Pharmacokinetics and Biopharmaceutics 17, 529–550 (1989). https://doi.org/10.1007/BF01071348
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01071348