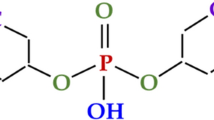
Abstract
The effect of additon of reactive phosphate rock (RPR — North Carolina) on the degree of acidulation of unreactive phosphate rocks (PRs — Nauru and Christmas Island A) during the manufacture of single superphosphate (SSP) was examined using32P in isotopic dilution studies. Acidulation of unreactive PR during SSP manufacture continued through denning, granulation and drying. Even after 3 hours drying, between 20 and 30% of the total P remained as free phosphoric acid in the reaction mixture. The addition of North Carolina phosphate rock (NCPR) to ex-den SSP reaction mixture (3:7 NCPR:SSP reaction mixture) preferentially consumed the free phosphoric acid remaining in the reaction mixture. This resulted in reduced acidulation of the unreactive PR in the reaction mixture and partial acidulation (10–23%) of the RPR. Hence the SSP-RPR mixture contains more residual, unreactive PR than is present in SSP.
The extent of partial acidulation of the RPR when mixed with SSP was determined by the nature of free acid remaining in the SSP reaction mixture, which in turn is affected by the type of unreactive PR used for SSP manufacture. The free acid in the Christmas Island A reaction mixture contained approximately 8 and 12 times as much Fe and Al respectively as that in the Nauru reaction mixture, and was only half as effective at converting the P in RPR to soluble P. Unless made with extended denning times and carefully chosen PR, SSP-RPR mixtures can contain (a) undesirable amounts of unreactive PR residues, and (b) low quality partially acidulated RPR, both of which have low agronomic value.
Similar content being viewed by others
References
AOAC (1975) Association of Official Agricultural Chemists, Official Methods of Analysis. 12th Edn., Sec.2.037 (AOAC; Washington DC)
Braithwaite AC and Crump RT (1980) Addition of North Carolina rock to ex-den superphosphate. Part 1. NZ Fertilizer Manufacturers Research Reports, 187–191
Johnson CM and Nishita H (1952) Micro-estimation of sulphur in plant materials, soils and irrigation waters. Analytical Chemistry 24: 736–742
Laing KR (1981) Two percent citric solubility of phosphate from reactive rock/superphosphate mixtures. NZ Fertilizer Manufacturers Research Reports, 325–328
Mackay AD, Syers JK and Gregg PEH (1984) A glasshouse comparison of 6 phosphate fertilizers. NZ J Exp Agric 12: 131–140
McClellan GH and Lehr JR (1969) Crystal and chemical investigation of nature apatites. Am Mineral 54: 1374–1391
McSweeney G, Charleston AG, Condron LM and Campbell AS (1985) Some properties of the residual apatite remaining in PAPR manufactured from North Carolina phosphate rock. Proceedings of 20th Technical Conference, NZ Fertilizer Manufactrurers Research Association, 393–410
Nunn RJ and Dee TP (1954) Superphosphate production: The influence of various factors on the speed of reaction and the composition of the product. J Sci Food Agric 5: 257–265
Quin BF (1981) Performance of reactivce phosphate rocks on irrigated and non-irrigated pasture. Proceedings of a Technical Workshop on the Potential of Phosphate Rock as a Direct Application Fertilizer in New Zealand, Occasional Report No. 3 pp. 13–20, Massey University, Palmerston North, New Zealand
Syers JK, MacKay AD, Brown MW and Currie LD (1986) Chemical and physical characteristics of phosphate rock materials of varying reactivity. J Sci Food Agric 37: 1057–1064
White MS (1971) Superphosphate from Christmas Island phosphate rock. NZ J Sci 14: 364–391
White MS (1974) The liquid phase of superphosphate. NZ J Sci 17: 171–182
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bolan, N.S., Hedley, M.J., Syers, J.K. et al. Single superphosphate-reactive phosphate rock mixtures. 1. Factors affecting chemical composition. Fertilizer Research 13, 223–239 (1987). https://doi.org/10.1007/BF01066446
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01066446