Abstract
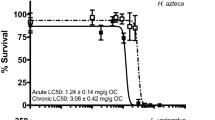
The addition of 100 (μg/L of Aroclor® 1242 (A1242) or 2,5,2′,5′-tetrachlorobiphenyl (TeCB) during 10 week chronic toxicity tests withHyalella azteca resulted in complete mortality. There were no effects on survival, growth, or reproduction after addition of 30 μg/L. Toxic effects were observed at tissue levels of between 30 and 180 μg/g on a wet weight basis, and tissue levels appear to be a better indicator of toxicity than levels in water. No toxic effects were observed after additions of up to 2,700 μg/L of the coplanar congener 3,4,3′,4′-TeCB.H. azteca has the ability to avoid accumulating in excess of 140 μg/g 3,4,3′,4′-TeCB. The amount taken up was proportional to the amount added in water up to 100 μg/L, but was constant at higher additions, possibly accounting for its relatively low toxicity. The low toxicity of the coplanar congener, as compared to the non-coplanar 2,5,2′,5′-TeCB, is in direct contrast to the high toxicity of coplanar PCB congeners to mammals and may be associated with slower rates of aromatic hydrocarbon metabolism in amphipods. Polychlorinated biphenyl levels measured in amphipods from Lake Ontario are approximately 100-fold below levels associated with toxicity inH. azteca, but are above levels which, through biomagnification up the food chain, lead to salmonid residues in excess of 2 μg/g, a tolerance limit for human consumption.
Similar content being viewed by others
References
Borgmann U, Ralph KM, Norwood WP (1989) Toxicity test procedures forHyalella azteca, and chronic toxicity of cadmium and pentachlorophenol toH azteca, Gammarus fasciatus, andDaphnia magna. Arch Environ Contam Toxicol 18:756–764
Borgmann U, Whittle DM (1983) Particle-size-conversion efficiency and contaminant concentrations in Lake Ontario biota. Can J Fish Aquat Sci 40:328–336
Cook DG, Johnson MG (1974) Benthic macroinvertebrates of the St. Lawrence Great Lakes. J Fish Res Board Can 31:763–782
DeFoe DL, Veith GD, Carlson RW (1978) Effects of Aroclor® 1248 and 1260 on the fathead minnow (Pimephales promelas). J Fish Res Board Can 35:997–1002.
Duke TW, Lowe JI, Wilson AJ (1970) A polychlorinated biphenyl (Aroclor 1254®) in the water, sediment, and biota of Escambia Bay, Florida. Bull Environ Contam Toxicol 5:171–180
Fox ME, Carey JH, Oliver BG (1983) Compartmental distribution of organochlorine contaminants in the Niagara River and the western basin of Lake Ontario. J Great Lakes Res 9:287–294
Kubiak TJ, Harris HJ, Smith LM, Schwartz TR, StaUing DL, Trick JA, Sileo L, Docherty DE, Erdman TC (1989) Microcontaminants and reproductive impairment of the Forster's tern on Green Bay, Lake Michigan-1983. Arch Environ Contam Toxicol 18:706–727
Landrum PF (1982) Uptake, depuration and biotransformation of anthracene by the scudPontoporeia hoyi. Chemosphere 11:1049–1057
Landrum PF, Scavia D (1983) Influence of sediment on anthracene uptake, depuration, and biotransformation by the amphipodHyalella azteca. Can J Fish Aquat Sci 40:298–305
Mauck WL, Mehrle PM, Mayer FL (1978) Effects of the polychlorinated biphenyl Aroclor® 1254 on growth, survival, and bone development in brook trout (Salvelinus fontinalis). J Fish Res Board Can 35:1084–1088
Mayer FL, Mehrle PM, Sanders HO (1977) Residue dynamics and biological effects of polychlorinated biphenyls in aquatic organisms. Arch Environ Contam Toxicol 5;501–511
McKinney JD, Chae K, McConnell EE, Birnbaum LS (1985) Structure-induction versus structure-toxicity relationships for polychlorinated biphenyls and related aromatic hydrocarbons. Environ Health Perspectives 60:57–68
McNulty WP, Becker GM, Cory HT (1980) Chronic toxicity of 3,4,3′,4′- and 2,5,2′,5′-tetrachlorobiphenyls in rhesus macaques. Toxicol Appl Pharmacol 56:182–190
Nebeker AV, Puglisi FA (1974) Effect of polychlorinated biphenyls (PCB's) on survival and reproduction ofDaphnia, Gammarus, andTanytarsus. Trans Am Fish Soc 103:722–728
Nebeker AV, Puglisi FA, DeFoe DL (1974) Effect of polychlorinated biphenyl compounds on survival and reproduction of the fathead minnow and flagfish. Trans Am Fish Soc 103:562–568
Niimi AJ, Oliver BG (1989) Assessment of relative toxicity of chlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls in Lake Ontario salmonids to mammalian systems using toxic equivalent factors (TEF). Chemosphere 18:1413–1423
Nimmo DR, Forester J, Heitmuller PT, Cook GH (1974) Accumulation of Aroclor® 1254 in grass shrimp (Palaemonetes pugio) in laboratory and field exposures. Bull Environ Contam Toxicol 11:303–308
Oliver BG, Niimi AJ (1988) Trophodynamic analysis of polychlorinated biphenyl congeners and other chlorinated hydrocarbons in the Lake Ontario ecosystem. Environ Sci Technol 22:388–397
Safe S (1984) Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs): Biochemistry, toxicology, and mechanism of action. CRC Crit Rev Toxicol 13:319–395
— (1987) Determination of 2,3,7,8-TCDD toxic equivalent factors (TEFs): Support for the use of the in vitro AHH induction assay. Chemosphere 16:791–802
Sonzogni WC, Swain WR (1984) Perspectives on human health concerns from Great Lakes contaminants. In Nriagu JO, Simmons MS (eds) Toxic contaminants in the Great Lakes. Wiley-Interscience, New York, pp 1–29
Tanabe S (1988) PCB problems in the future: Foresight from current knowledge. Environ Pollut 50:5–28
Varanasi U, Reichert WL, Stein JE, Brown DW, Sanborn HR (1985) Bioavailability and biotransformation of aromatic hydrocarbons in benthic organisms exposed to sediment from an urban estuary. Environ Sci Technol 19:836–841
Whittle DM, Fitzsimons JD (1983) The influence of the Niagara River on contaminant burdens of Lake Ontario Biota. J Great Lakes Res 9:295–302
Winnell MH, Jude DJ (1987) Benthic community structure and composition among rocky habitats in the Great Lakes and Keuka Lake, New York. J Great Lakes Res 13:3–17
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Borgmann, U., Norwood, W.P. & Ralph, K.M. Chronic toxicity and bioaccumulation of 2,5,2′,5′- and 3,4,3′,4′-tetrachlorobiphenyl and Aroclor® 1242 in the amphipodHyalella azteca . Arch. Environ. Contam. Toxicol. 19, 558–564 (1990). https://doi.org/10.1007/BF01059075
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01059075