Abstract
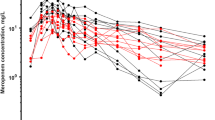
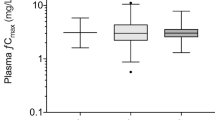
Moxalactam pharmacokinetics at steady state was examined in a group of 40 patients with presumed or proven abdominal sepsis. Mean steady-state serum concentrations ranged from 27.0 to 211.0 mcg/ml and correlated inversely with creatinine clearance (r=0.91, p<0.0001). Terminal half-life ranged from 1.27 to 8.27 hr and reflected the varying renal function of the patients. Moxalactam total body clearance (CL)displayed excellent correlation with creatinine clearance as 92%(r 2×100)of the variance in clearance could be accounted for by renal function (p< 0.0001). Pharmacokinetic parameters were estimated using noncompartmental analysis based on statistical moment theory. Noncompartmentally determined CLwas in agreement with CLdetermined by nonlinear least squares regression (r=0.99, p< 0.0001).Moxalactam total body clearance is best predicted from creatinine clearance corrected for body surface area.
Similar content being viewed by others
References
T. Yoshida, S. Matsuura, M. Mayama, Y. Kameda, and S. Kuwuhara. Moxalactam (6059-S), a novel 1-oxa-β-lactam with an expanded antibacterial spectrum: laboratory evaluation.Antimicrob. Agents Chemother. 17:302–312 (1980).
J. J. Schentag, P. B. Wels, D. P. Reitberg, P. Walczak, M. J. Hawkins-Van Tyle, and R. J. Lascola. A randomized clinical trial of moxalactam alone versus tobramycin plus clindamycin in abdominal sepsis. Ann. Surg. (in press).
M. D. Reed, J. S. Bertino, S. C. Aronoff, W. T. Speck, and J. L. Blumer. Evaluation of moxalactam.Clin. Pharmacy 1:124–134 (1982).
W. K. Bolton, W. M. Scheid, D. A. Spyker, T. L. Overby, and M. A. Sande. Pharmacokinetics of moxalactam in subjects with various degrees of renal dysfunction.Antimicrob. Agents Chemother. 18:933–938 (1980).
M. Lam, C. V. Manion, and A. W. Czerwinsky. Pharmacokinetics of moxalactam in patients with renal insufficiency.Antimicrob. Agents Chemother. 19:461–464 (1981).
G. R. Aronoff, R. S. Sloan, and F. C. Luft. Pharmacokinetics of moxalactam in patients with normal and impaired renal function.J. Infect. Dis. 145:365 (1982).
A. Leroy, G. Humbert, and J. P. Fillastre. Pharmacokinetics of moxalactam in subjects with normal and impaired renal function.Antimicrob. Agents Chemother. 19:965–971 (1981).
K. S. Israel, M. R. Black, G. L. Brier, J. D. Wolny, and K. A. DeSante. Single and multiple dose pharmacokinetics of moxalactam in normal subjects.Antimicrob. Agents Chemother. 22:94–102 (1982).
P. Garzone, J. Lyon, V. L. Yu, J. Zuravleff, W. Diven, and W. Pasculle. Steady-state moxalactam kinetics: comparisons with other cephalosporins.Clin. Pharmacol. Ther. 30:86–94 (1981).
M. Gibaldi and D. Perrier. Drugs and the pharmaceutical sciences. InPharmacokinetics, Vol. 1, J. Swarbrick (ed.), Marcel Dekker, New York, 1975, Chaps. 2 and 5.
W. J. Jusko. Guidelines for collection and pharmacokinetic analysis of drug disposition Data. InApplied Pharmacokinetics, J. J. Schentag and W. J. Jusko (eds.), Applied Therapeutics, Inc., San Francisco, 1980, pp. 639–680.
C. Daniel and F. S. Wood.Fitting Equations to Data. Wiley-Interscience, New York, 1971.
H. G. Boxenbaum, S. Riegelman, and R. M. Elashoff. Statistical estimations in pharmacokinetics.J. Pharmacokin. Biopharm. 2:123–148 (1974).
K. C. Yeh and K. C. Kwan. A comparison of numerical integrating algorithms by trapezoidal, LaGrange, and Spline approximation.J. Pharmacokin. Biopharm. 6:79–98 (1978).
J. A. Ziemniak, D. A. Chiarmonte, D. J. Miner, and J. J. Schentag. HPLC determination of D and L moxalactam in human serum and urine.J. Pharm. Sci. 71:399–402 (1982).
J. Kurihara, K. Matsumoto, Y. Uzuka, H. Shishido, T. Yamada, T. Yoshida, T. Oguma, Y. Kimura, and Y. Tochino. Clinical evaluation of 6059-S, a new active oxacephem. InProc. 11th Int. Congress of Chemotherapy and the 19th Intersci. Conf. Antimicrobial Agents and Chemotherapy, Vol. 1, J. D. Nelson and C. Grassi (eds.), Am. Soc. Microbiol, Washington D.C., 1979, p. 112.
J. N. Parsons, J. M. Romano, and M. E. Levison. Pharmacology of a new oxa-β-lactam (LY127935) in normal volunteers.Antimicrob. Agents Chemother. 17:226–228 (1981).
K. Yamaoka, Y. Tanigawara, T. Nakagawa, and T. Uno. A pharmacokinetic analysis program (multi) for microcomputer.J. Pharm. Dyn. 4:879–885 (1981).
M. Gibaldi and D. Perrier. Drugs and the pharmaceutical sciences. InPharmacokinetics, Vol. 15, J. Swarbrick (ed.), Marcel Dekker, New York, 1982, pp. 409–416.
B. Carnahan, H. A. Luther, and J. O. Welkes.Applied Numerical Methods. Wiley, New York, 1969, pp. 27–34.
K. Siersbaek-Nielsen, J. M. Hansen, J. Kampmann, and M. Kristensen. Rapid evaluation of creatinine clearance.Lancet 1:1133 (1971).
M. M. Chrymko and J. J. Schentag. Creatinine clearance predictions in acutely ill patients.Am. J. Hosp. Pharm. 38:837–840 (1981).
R. Luthy, R. Wise, A. Bonetti, and J. Blaster. Comparative multiple does pharmacokinetics of cefotaxime and moxalactam (LY 127935). 20th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, 1980, Am. Soc. for Microbiology, Washington D.C., Abst. No. 239.
O. V. Martinez, J. U. Levi, A. Livingstone, T. I. Malinin, R. Zeppa, D. Hutson, and N. Einhorn. Biliary excretion of moxalactam.Antimicrob. Agents Chemother. 20:231–234 (1980).
L. Z. Benet and R. L. Galeazzi. Non-compartmental determination of the steady-state volume of distribution.J. Pharm. Sci. 68:1071–1074 (1979).
D. Perrier and M. Mayersohn. Non-compartmental determination of the steady-state volume of distribution for any mode of administration.J. Pharm. Sci. 71:372–373 (1982).
I. L. Smith and J. J. Schentag. Non-compartmental determination of the steady-state volume of distribution during multiple dosing.J. Pharm. Sci. (in press).
Author information
Authors and Affiliations
Additional information
Supported by a grant from Eli Lilly Company and in part by NIGMS Grant 20852 from the National Institutes of Health.
Rights and permissions
About this article
Cite this article
Swanson, D.J., Reitberg, D.P., Smith, I.L. et al. Steady-state moxalactam pharmacokinetics in patients: Noncompartmental versus two-compartmental analysis. Journal of Pharmacokinetics and Biopharmaceutics 11, 337–353 (1983). https://doi.org/10.1007/BF01058954
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01058954