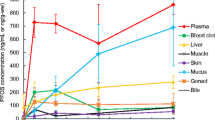
Abstract
Juvenile starry flounder (Platichthys stellatus) and rock sole (Lepidopsetta bilineata) were force-fed 56 μCi each of 1-3H-naphthalene dissolved in salmon oil. Values for radioactivity associated with naphthalene and the metabolite fraction were determined for various tissues and body fluids. Results show that these pleuronectids extensively metabolize dietary naphthalene. The rates of decline in naphthalene concentrations (expressed as disintegrations per minute per milligram of dry tissue) were greater than the rates of decline in metabolite concentrations (dpm/mg) in liver, blood, and skin; therefore, relative proportion of metabolites to naphthalene increased with time and at 168 hr after the initiation of the naphthalene-exposure, more than half of the total radioactivity in both species of fish was associated with the metabolites.
Profiles of metabolites in liver, skin, and bile were obtained using thin-layer chromatography. 1,2-Dihydro-1,2-dihydroxynaphthalene constituted 38.7 and 39.7%, respectively, of the total extracted metabolites in livers of the naphthalene-exposed rock sole and starry flounder at 24 hr, whereas the bile from both species contained primarily (>90%) conjugates. From 24 to 168 hr, a significant (P < 0.05) decrease in the proportion of the dihydrodiol derivative and a concomitant increase in the proportion of conjugates—specifically, sulfate/glucoside fraction-were observed with livers of both rock sole and starry flounder. No significant change occurred in the spectrum of biliary metabolites with time. The pattern of metabolites in skin of both species was qualitatively similar to that in liver; however, the proportion of the dihydrodiol was greater in skin than in liver at 24 hr.
When naphthalene (56μCi) dissolved in salmon oil was administered to starry flounder via intraperitoneal injection, the extent of biotransformation was less than after dietary exposure. Moreover, metabolites in the livers of the fish in the injection study were predominantly (76.7% of total extracted metabolites) non-conjugates at 24 hr. Once again, from 24 to 168 hr, an increase in the proportion of the sulfate/glucoside fraction and a concomitant decrease in the proportion of the dihydrodiol was observed with liver.
These studies demonstrate that the extent of biotransformation of naphthalene and the types of metabolites remaining in tissues (e.g., liver) of flatfish are greatly influenced by both the mode of exposure and the time elapsed after the exposure is initiated. It appears therefore, that different exposures (e.g., in water, food, or sediment) of pleuronectids to polycyclic aromatic hydrocarbons may result in different degrees of alteration in genetic material because of variability in accumulation of non-conjugated metabolites, some of which are implicated in covalent binding with DNA in terrestrial mammals.
Similar content being viewed by others
References
Anderson, J. W.: Responses to sublethal levels of petroleum hydrocarbons: Are they sensitive indicators and do they correlate with tissue contamination? In D. A. Wolfe (ed).: Fate and effects of petroleum hydrocarbons in marine organisms and ecosystems. p. 95. New York: Pergamon Press (1977).
Barrington, E. J. W.: The alimentary canal and digestion. In M. E. Brown (ed.): The physiology of fishes Vol. 1, p. 109. New York: Academic Press (1957).
Bend, J. R., and M. O. James: Xenobiotic metabolism in marine and freshwater species. In D. C. Malins and J. H. Sargent (ed.): Biochemical and biophysical perspectives in marine biology Vol. IV. p. 125. New York: Academic Press (1978).
Boyland, E., G. S. Ramsay, and P. Sims: Metabolism of polycyclic compounds. 18. The secretion of metabolites of naphthalene, 1∶2-dihydronaphthalene, and 1∶2-epoxy-1∶2∶3∶4-tetrahydronaphthalene in rat bile. Biochem. J.78, 376 (1961).
Boyland, E., and J. B. Solomon: Metabolism of polycyclic compounds. 10. Estimation of metabolites of naphthalene by paper chromatography. Biochem. J.63, 679 (1956).
Brookes, P.: Mutagenicity of polycyclic aromatic hydrocarbons. Mutation Res.39, 257 (1977).
Burke, M. D., H. Vadi, B. Jernstrom, and S. Orrenius: Metabolism of benzo(a)pyrene with isolated hepatocytes and the formation and degradation of DNA-binding derivatives. J. Biol. Chem.252, 6424 (1977).
Collier, T. K.: Disposition and metabolism of naphthalene in rainbow trout (Salmo gairdneri). M.S. Thesis, University of Washington, Seattle (1978).
Collier, T. K., L. C. Thomas, and D. C. Malins: Influence of environmental temperature on disposition of dietary naphthalene in coho salmon (Oncorhynchus kisutch): Isolation and identification of individual metabolites. Comp. Biochem. Physiol.61c, 23 (1978).
Corner, E. D. S., R. P. Harris, C. C. Kilvington, and S. C. M. O'Hara: Petroleum compounds in the marine food web: Short-term experiments on the fate of naphthalene inCalanus. J. Mar. Biol. Assoc. U.K.56, 121 (1976).
Daniel, P. M., and O. E. Pratt: Metabolism of labeled carcinogenic hydrocarbons in rats. Nature215, 1142 (1967).
DePierre, J. W., and L. Ernster: The metabolism of polycyclic hydrocarbons and its relationship to cancer. Biochim. Biophys. Acta.473, 149 (1978).
Dixit, D., and J. W. Anderson: Distribution of naphthalenes within exposedFundulus similus and correlations with stress behavior. In: Proceedings 1977 Oil Spill Conference (Prevention, Behavior, Control, Cleanup), p. 633. Washington, DC: American Petroleum Institute (1977).
Gaurino, A. M., P. M. Briley, J. B. Anderson, M. A. Kinter, S. Schneiderman, L. D. Klipp, and R. H. Adamson: Renal and hepatic excretion of foreign compounds bySqualus acanthias. Bull. Mt. Desert Island Biol. Lab.12, 41 (1972).
Geiszler, P. C., B. J. Grantham, and G. J. Blomquist: Fate of labeledn-alkanes in the blue crab and stripped mullet. Bull. Environ. Contam. Toxicol.17, 463 (1977).
Gibaldi, M., and D. Perrier: Route of administration and drug disposition. Drug Metab. Rev.3, 185 (1974).
Hanson, S. W. F., and J. Olley: Application of the Bligh and Dyer method of lipid extraction to tissue homogenates. Biochem. J.89, 101 (1963).
Hodgins, H. O., B. B. McCain, and J. W. Hawkes: Marine fish and invertebrate diseases, host disease resistance, and pathological effects of petroleum. In: D. C. Malins (ed.): Effects of petroleum on arctic and subarctic marine environments and organisms Vol. II, p. 95. New York: Academic Press (1977).
Jones, P. W., and R. I. Freudenthal (ed.): Carcinogenesis-A Comprehensive Survey, Vol. 3. Polynuclear Aromatic Hydrocarbons. Second International Symposium on Analysis, Chemistry, and Biology. New York: Raven Press (1978).
Kapitulnik, J., W. Levin, A. H. Conney, H. Yagi, and D. M. Jerina: Benzo[a]pyrene 7,8-dihydrodiol is more carcinogenic than benzo[a]pyrene in newborn mice. Nature266, 378 (1977).
Levin, W., A. W. Wood, H. Yagi, D. M. Jerina, and A. H. Conney: (±)-trans-7,8-Dihydroxy-7,8-dihydrobenzo(a)pyrene: A potent skin carcinogen when applied topically to mice. Proc. Natl. Acad. Sci. USA73, 3867 (1976).
Magee, P. N.: Activation and inactivation of chemical carcinogens and mutagens in the mammal. In P. N. Campbell and F. Dickens, (ed.): Essays in Biochemistry Vol. 10, p. 105. London: Academic Press (1974).
Malins, D. C.: Metabolism of aromatic hydrocarbons in marine organisms. Ann. N.Y. Acad. Sci.298, 482 (1977).
Malins, D. C., T. K. Collier, L. C. Thomas, and W. T. Roubal: Metabolic fate of aromatic hydrocarbons in aquatic organisms: Analysis of metabolites by thin-layer chromatography and high-pressure liquid chromatography. Intl. J. Environ. Anal. Chem. (In press 1978).
Melancon, M. J., and J. J. Lech: Distribution and elimination of naphthalene and 2-methylnaphthalene in rainbow trout during short- and long-term exposures. Arch. Environ. Contam. Toxicol.7, 207 (1978).
McCain, B. B., K. V. Pierce, S. R. Wellings, and B. S. Miller: Hepatomas in marine fish from an urban estuary. Bull. Environ. Contam. Toxicol.18, 1 (1977).
Miller, B. S.: Stomach contents of adult starry flounder and sand sole in east sound, Orcas Island, Washington. J. Fish. Res. Bd. Canada24, 2515 (1967).
Miller, E. C., and J. A. Miller: Biochemical mechanisms of chemical carcinogenesis. In: H. Busch, (ed.): The Molecular Biology of Cancer p. 377. New York: Academic Press (1974).
Norwich, K. H.: Molecular Dynamics in Biosystems. New York: Pergamon Press (1977).
Roubal, W. T., T. K. Collier, and D. C. Malins: Accumulation and metabolism of carbon-14 labeled benzene, naphthalene, and anthracene by young coho salmon (Oncorhynchus kisutch). Arch. Environ. Contam. Toxicol.5, 513 (1977).
Roubal, W. T., S. I. Stranahan, and D. C. Malins: The accumulation of low molecular weight aromatic hydrocarbons of crude oil by coho salmon (Oncorhynchus kisutch) and starry flounder (Platichthys stellatus). Arch. Environ. Contam. Toxicol.7, 237 (1978).
Savolainen, H.: Some aspects of the mechanisms by which industrial solvents produce neurotoxic effects. Chem.-Biol. Interactions18, 1 (1977).
Schmeltz, I., J. Tosk, J. Hilfrich, N. Hirota, D. Hoffmann, and E. L. Wynder: Bioassays of naphthalene and alkylnaphthalenes for co-carcinogenic activity Relation to tobacco carcinogenesis. In: P. W. Jones and R. I. Freudenthal (eds.): Carcinogenesis, polynuclear aromatic hydrocarbons Vol. 3 p. 47. New York: Raven Press (1978).
Sieber, S. M., and R. H. Adamson: The metabolism of xenobiotics by fish. In D. V. Parke and R. L. Smith, (ed.): Drug metabolism—from microbe to man P. 233. London: Taylor and Francis, Ltd. (1976).
Sims, P., and P. L. Grover: Epoxides in polycyclic aromatic hydrocarbon metabolism and carcinogenesis. In G. Klein, S. Weinhouse, and A. Hadden (ed.): Advances in cancer research Vol. 20, p. 165. New York: Academic Press (1974).
Statham, C. N., C. R. Elcombe, S. P. Szyjka, and J. J. Lech: Effect of polycyclic aromatic hydrocarbons on hepatic microsomal enzymes and disposition of methylnaphthalene in rainbow troutin vivo. Xenobiotica8, 65 (1978).
Swaisland, A. J., A. Hewer, K. Pal, G. R. Keysell, J. Booth, P. L. Grover, and P. Sims: Polycyclic hydrocarbon epoxides: The involvement of 8,9-dihydro-8,9-dihydroxybenz(a)anthracene 10,11-oxide in reactions with the DNA of benz(a)anthracene-treated hamster embryo cells. FEBS Lett.47, 34 (1974).
Varanasi, U., and D. C. Malins: Metabolism of petroleum hydrocarbons: Accumulation and biotransformation in marine organisms. In: D. C. Malins (ed.): Effects of petroleum on arctic and subarctic marine environments and organisms Vol. II, p. 175. New York: Academic Press (1977).
Varanasi, U., and D. Markey: Uptake and release of lead and cadmium in skin and mucus of coho salmon (Oncorhynchus kisutch). Comp. Biochem. Physiol.60C, 187 (1978).
Varanasi, U., M. Uhler, and S. I. Stranahan: Uptake and release of naphthalene and its metabolites in skin and epidermal mucus of salmonids. Toxicol. Appl. Pharmacol.44, 277 (1978).
Wood, A. W., W. Levin, D. Ryan, P. E. Thomas, H. Yagi, H. D. Mah, D. R. Thakker, D. M. Jerina, and A. H. Conney: High mutagenicity of metabolically activated chrysene 1,2 dihydrodiol: Evidence for bay region activation of chrysene. Biochem. Biophys. Res. Commun.78, 847 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Varanasi, U., Gmur, D.J. & Treseler, P.A. Influence of time and mode of exposure on biotransformation of naphthalene by Juvenile starry flounder (Platichthys stellatus) and rock sole (Lepidopsetta bilineata). Arch. Environ. Contam. Toxicol. 8, 673–692 (1979). https://doi.org/10.1007/BF01054869
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01054869