Abstract
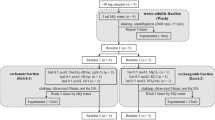
The dissolution of phosphate rock (PR) in soil was determined by measuring the amount of either the dissolved inorganic phosphate (Pi) and Ca or the residual P and Ca remaining as undissolved PR in the soil. The dissolved Pi was extracted by either 0.5M NaOH or 0.5M NaHCO3 and the dissolved Ca was extracted using either 0.5M BaCl2/triethanolamine (TEA) or 1M NH4OAc. Undissolved PR was extracted by 1M HCl following the extraction with either 0.5M NaOH or 0.5M BaCl2/TEA.
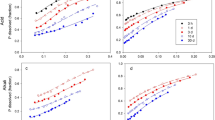
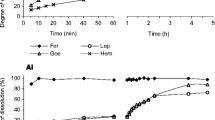
From the mixture of PR with dry soil, the 0.5M NaOH extracted between <0.1 and 4.7% of the undissolved Pi and the 0.5M BaCl2/TEA extracted between 2.8 to 7.8% of the undissolved Ca. From PR incubated with moist soil, these reagents extracted most of the dissolved Pi or Ca (95 to 99%). The 0.5M NaHCO3 extracted between < 0.1 to 4.8% of the undissolved Pi and the 1M NH4OAc extracted between 5.2 to 10.5% of the undissolved Ca from the PR/dry soil mixture. However the extraction of dissolved Pi and Ca from PR incubated with moist soil by these reagents was incomplete (30 to 80%). The 0.5M NaHCO3 and 1M NH4OAc extractants tended to underestimate the extent of PR dissolution when the levels of dissolved Pi and Ca were high. The difference between the amounts of 0.5M NaOH extractable Pi (ΔP) and 0.5M BaCl2/TEA extractable Ca (ΔCa) in the PR-treated soil and in the soil alone gave a more accurate estimate of the extent of PR dissolution in laboratory incubated soils. However, under field conditions where the Pi and Ca released during the dissolution of PR can be removed by plant uptake and leaching, these two methods, which measure the amount of the dissolution products, may underestimate the extent of dissolution. Under these conditions, the amount of residual P or Ca extracted by 1M HCl is recommended as the best estimate of the amount of undissolved PR remaining in soil.
Similar content being viewed by others
References
Axelrod S and Greidinger D (1970) Phosphate solubility tests — interference of some accessory minerals. J Sci Food Agric 30: 153–157
Apthorp JN, Hedley MJ and Tillman RW (1987) The effects of nitrogen fertilizer form on the plant availability of phosphate from soil, phosphate rock and mono-calcium phosphate. Fert Res 12: 269–284.
Barrow NJ and Shaw TC (1976) Sodium bicarbonate as an extractant for soil phosphate. 1. Separation of the factors affecting the amount of phosphate displaced from soil from those affecting secondary adsorption. Geoderma 16: 91–107
Bascomb CL (1964) Rapid method for the determination of cation exchange capacity of calcareous and noncalcareous soils. J Sci Food Agric 15: 821–823
Blakemore LC, Searle PL and Daly BK (1987) Methods for chemical analysis of soils. NZ Soil Bureau Scientific Report 80
Bolland MDA, Bowden JW, D'Antuono MF and Gilkes RJ (1984) The current and residual value of superphosphate, Christmas Island C-grade ore, and Calciphos as fertilizers for a subterranean clover pasture. Fert Res 5: 335–354
Bremmner JM and Tabatabai MA (1971) Use of automated combustion techniques for total carbon, total nitrogen and total sulphur analysis of soils. In: Instrumental methods for analysis of soils and plant tissue (Ed. Walsh L M), Soil Sci Soc Am, Madison
Cano Ruz J and Talibudeen O (1957) Surface properties of phosphate materials by isotopic exchange. 1. Rock phosphate. J Sci Food Agric 8: 305–308
Chien SH (1977) Dissolution rates of phosphate rocks. Soil Sci Soc Am J 41: 656–657
Chien SH, Hammond LL and Leon LA (1987) Long-term reactions of phosphate rocks with an oxisol of Columbia. Soil Sci 144: 257–265
Edmeades DC (1982) Effects of lime on effective cation exchange capacity and exchangeable cations in a range of New Zealand soils. NZ J Agric Res 25: 27–33
Gillman GA, Bruce RC, Davey BG, Kimble JM, Searle PL and Skjemstad JO (1983) A comparison of methods used for determination of cation exchange capacity. Comm Soil Sci Pl Anal 14: 1005–1014
Hughes JC and Gilkes RJ (1984) The effect of chemical extractant on the estimation of rock phosphate fertilizer dissolution. Aust J Soil Res 22: 475–481
Hughes JC and Gilkes RJ (1986) The effect of rock phosphate properties on the extent of fertilizer dissolution in soils. Aust J Soil Res 24: 209–217
Khasawneh FE and Doll EC (1978) The use of phosphate rock for direct application to soils. Adv Agron 30: 159–206
Kinniburgh DG, Syers JK and Jackson ML (1975) Specific adsorption of trace amounts of calcium and strontium by hydrous oxides of iron and aluminium. Soil Sci Soc Am J 39: 464–470
Kirk GJD and Nye PH (1986) The dissolution and dispersion of dicalcium phosphate dihydrate in soils. IV Experimental evaluation of the model for particles. J Soil Sci 37: 525–528
Lee A, Watkinson JH, Orbell G, Bagyaraj J and Lauren DR (1987) Factors influencing the dissolution of phosphate rock and oxidation of elemental sulphur in some New Zealand soils. NZ J Agric Res 30: 373–385
Mackay AD (1984) Field evaluation of Chatham Rise Phosphorite. Ph.D thesis, Massey University, Palmerston North, New Zealand
Mackay AD, Gregg PEH and Syers JK (1984) Field evaluation of Chatham Rise Phosphorite as a phosphatic fertilizer for pasture. NZ J Agric Res 27: 65–82
Mackay AD and Syers JK (1986) Effect of phosphate, calcium and pH on the dissolution of a phosphate rock in soil. Fert Res 10: 175–184
Mackay AD, Syers JK, Tillman RW and Gregg PEH (1986) A simple model to describe the dissolution of phosphate rock in soils. Soil Sci Soc Am J 50: 291–296
McClellan GH (1978) Mineralogy and reactivity of phosphate rock. Proceedings of a seminar on phosphate rock for direct application, International Fertilizer Development Centre, Muscle Shoals, Alabama. pp 57–81
McKeague JA and Day JH (1966) Dithionate and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Canadian J Soil Sci 46: 13–22
McLaughlin JR, Ryden JC and Syers JK (1977) Development and evaluation of a kinetic model to describe phosphate sorption by hydrous ferric oxide gel. Geoderma 18: 295–307
Murphy J and Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27: 31–36
O'Connor PW and Syers JK (1975) Comparison of methods for the determination of total P in waters containing particulate materials. J Environ Qual 4: 347–350
Olsen SR, Cole CV, Watanabe FS and Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US Dept Agric Circ, 339
Ozanne PG, Kirton DJ and Shaw TC (1961) The loss of phosphorus from sandy soils. Aust J Agric Res 12: 409–415
Parfitt RL (1980) Chemical properties of variable charge soils. In: ‘Soils of Variable Charge’ (Ed. B K G Theng), NZ Soil Sci Soc, Lower Hutt, New Zealand.
Rajan SSS (1983) Effect of sulphur content of phosphate rock/sulphur granules on the availability of phosphate to plants. Fert Res 4: 287–296
Ryden JC, McLaughlin JR and Syers JK (1977) Time-dependent sorption of phosphate by soils and hydrous ferric oxides. J Soil Sci 28: 585–595
Silverman SR, Fuyat RK and Weiser JD (1962) Quantitative determination of calcite associated with carbonatebearing apatites. Am Mineralogist 37: 211–222
Smyth TJ and Sanchez PA (1982) Phosphate rock dissolution and availability in Cerrado soils as affected by phosphorus sorption capacity. Soil Sci Soc Am J 46: 339–345
Sorn-srivichai P, Tillman RW, Syers JK and Cornforth IS (1984) The effect of soil pH on Olsen bicarbonate phosphate values. J Sci Food Agric 35: 257–264
Syers JK and MacKay AD (1986) Reactions of Sechura phosphate rock and single superphosphate in soil. Soil Sci Soc Am J 50: 480–485
Syers JK and MacKay AD, Brown MW and Currie LD (1986) Chemical and Physical characteristics of phosphate rock materials of varying reactivity. J Sci Food Agric 37: 1057–1064
Syers JK, Smillie GW and Williams JDH (1972) Calcium fluoride formation during extraction of calcareous soils with fluoride. 1. Implications to inorganic P fractionation schemes. Soil Sci Soc Am Proc 36: 20–25
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bolan, N.S., Hedley, M.J. Dissolution of phosphate rocks in soils. 1. Evaluation of extraction methods for the measurement of phosphate rock dissolution. Fertilizer Research 19, 65–75 (1989). https://doi.org/10.1007/BF01054677
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01054677