Abstract
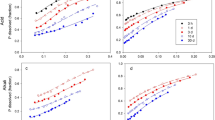
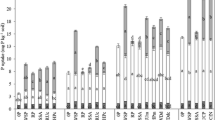
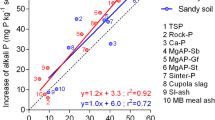
PAPR was made by partial acidulation of North Carolina phosphate rock with H3PO4. The PAPRs were incubated in bands in columns of two soils of contrasting P retention. The columns were sampled after freezing and sectioning with a cryomicrotome. The movement of P in soil incubated with33P-labelled PAPR was followed by autoradiography of polished epoxy impregnated sections of the freeze-dried soil column. PAPR solubility was also studied by a sequential dialysis process using distilled deionised water. The acid solution resulting from the dissolution of monocalcium phosphate (MCP) in PAPR moved into the surrounding soil, solubilizing soil minerals and creating a low-pH front with a high concentration of P. Depending on the soil, phosphorus moved 6–14 mm away from the fertilizer/soil interface by mass flow and diffusion in two days. The increase in 0.5 M NaOH extractable P above that of untreated soil showed a maximum at the same position as the pH minimum in the soil. In both soils, the total P movement from the fertilizer band after a two day period for 50% PAPR was comparable to that for 100% acidulation (≡triple superphosphate), indicating that acidulations above 50% did not necessarily increase the movement of soluble P from the fertilizer pellet. Variations in pH in the fertilizer-affected soil could be explained by the net balance of acidity resulting from incoming acid P solution and release of OH− during P sorption. The rock residue exhibited a transient loss in solubility which was reversed on subsequent dissolution, suggesting a possible surface alteration.
Similar content being viewed by others
References
Barrow NJ (1989) Surface reactions of phosphate in soil. Agric Sci 2: 33–37
Barrow NJ (1989) The reaction of plant nutrients and pollutants with soil. Aust J Soil Res 27: 475–492
Benbi DK and Gilkes RJ (1987) The movement of P from superphosphate grains and its availability to plants. Fert Res 12: 21–36
Bowden JW Posner AM and Quirk JP (1977) Ionic adsorption on variable charge mineral surfaces: Theoretical charge development and titration curves. Aust J Soil Res 15: 121–136
Bowden JW, Nagarajah S, Barrow NJ, Posner AM and Quirk JP (1980) Describing the adsorption of phosphate citrate and selenite on a variable charge mineral surface. Aust J Soil Res 18: 49–60
Braithwaite AC (1986) A comparison of fertilizers made by partially and fully acidulating phosphate rocks with phosphoric acid. NZJ Tech 2: 37–42
Brown LE and Lehr JR (1959) Application of phase rule to the behavior of monocalcium phosphate monohydrate in soils. Soil Sci Soc Am Proc 23: 7–11
Charlston AG, Condron LM and Brown IWM (1989) The nature of residual apatites remaining after partial acidulation of phosphate rocks with phosphoric acid and sulphuric acids. Fert Res 18: 257–273
Chien SH and Black CA (1976) Free energy of formation of carbonate apatite in some phosphate rocks. Soil Sci Soc Am Proc 40: 234–239
Davies CW (1962) Ion Associations. Butterworths, London
Garbouchev IP (1981) The manufacture and agronomic efficiency of a partially acidulated phosphate rock fertilizer. Soil Sci Soc Am J 45: 970–974
Hagin J and Katz S (1985) Effectiveness of partially acidulated phosphate rock as a source to plants in calcareous soils. Fert Res 8: 117–127
Junge A and Werner W (1989) Investigations on reactions of phosphorus compounds in partially acidulated phosphate rock and fertilizer effectiveness. Fert Res 20: 129–134
Khasawneh FE and Doll EC (1978) The use of phosphate rock for direct application to soils. Adv Agron 30: 159–206
Kirk GJD and Nye PH (1986) A simple model for predicting the rates of dissolution of sparingly soluble calcium phosphate in soil. I. The basic model. J Soil Sci 37: 529–540
Lehr JR, Brown WE and Brown EH (1959) Chemical behavior of monocalcium phosphate monohydrate in soils. Soil Sci Soc Am Proc 23: 3–7
Lindsay WL and Vlek PLG (1977) Phosphate minerals, In Minerals in soil environments, JB Dixon and SB Weed (ed) Soil Science Society of America, Madison WI, pp 639–688
Lindsay WL (1979) Chemical Equilibria in Soils. John Wiley, New York
Lindsay WL and Stephenson HF (1959) Nature of the reactions of monocalcium phosphate monohydrate in soils. 1. The solution that reacts with the soil. Soil Sci Soc Am Proc 23: 12–18
Lindsay WL and Stephenson HF (1959) Nature of the reaction of monocalcium phosphate monohydrate in soils. II. Dissolution and precipitating reactions involving iron, aluminum, manganese and calcium. Soil Sci Soc Am Proc 23: 18–22
Lindsay WL and Stephenson HF (1959) Nature of the reaction of moncalcium phosphate monohydrate in soils: Repeated reactions with metastable triple point solution. Soil Sci Soc Am Proc 23: 440–445
McLean EO and Balam BS (1967) Partially acidulated rock phosphate as a source of phosphorus to plants. III Uptake by corn from soils of different calcium status. Soil Sci Soc Am Proc 31: 811–814
McLean EO and Wheeler RW (1964) Partially acidulated rock phosphate as a source of phosphorus to plants. I. Growth chamber studies. Soil Sci Soc Am Proc 28: 545–550
McLean EO, Wheeler RW, Watson JD (1965) Partially acidulated phosphate rock as a source of phosphorus to plants II. Growth and field corn studies. Soil Sci Soc Am Proc 29: 625–628
McLean EO and Ssali H (1977) Effects of phosphorus rate and form in combination with lime and gypsum on yields and compositions of German millet and alfalfa from highly weathered soils. Soil Sci 123: 155–164
Marwaha BC (1983) Partially acidulated rock phosphate as a source of fertilizer phosphorus with special reference to high P-fixing acid soils — a review. Proc Ind Natn Sci Acad B49: 436–446
Misra UK and Panda N (1969) Evaluation of partially acidulated rock phosphate in a lateritic soil. Ind J Agric Sci 39: 353–351
Mokwunye AU and Chien SH (1980) Reactions of partially acidulated rock with soils from the tropics. Soil Sci Soc Am J 44: 477–482
Nye PH (1979) Diffusion of ions and uncharged solutes in soils and soil clays. Adv Agron 31: 225–271
Parfitt RL (1989) Phosphate reactions with natural allophane, ferrihydrite and goethite. J Soil Sci 40: 359–369
Rajan SSS (1975) Mechanism of phosphate adsorption by allophane clays. NZ J Sci 18: 93–101
Rajan SSS (1982) Availability to plants of phosphate from ‘biosupers’ and partially acidulated phosphate rock. NZ J Agric Res 25: 355–361
Resseler H and Werner W (1989) Properties of unreacted rock residues in partially acidulated rocks affecting their reactivity. Fert Res 20: 135–143
Saunders WMH (1965) Phosphate sorption by New Zealand soils and its relationship to free sesquioxides, organic matter and other soil properties. NZ J Agr Res 8: 30–57
Stephen RC and Condron LM (1986) An assessment of the agronomic efficiency of partially acidulated phosphate rock fertilizers. Fert Res 10: 269–282
Syers JK and Mackay AD (1986) Reactions of Sechura phosphate rock and single superphosphate in soil. Soil Sci Soc Am J 50: 480–485
Terman GL and Allen SE (1967) Response of corn to phosphorus in under acidulated phosphate rock and rock-superphosphate fertilizers. J Agri Food Chem 15: 354–358
Wada K (1977) Allophane and imogolite, In Minerals in soil environments, JB Dixon and SB Weed (ed) Soil Science Society of America. Madison, WI, pp 603–638
White RE (1980) Retention and release of phosphate by soil and soil constituents. In: Soils and Agriculture. Critical reports on applied chemistry. Vol. 2. PB Tinker (ed) Blackwell Scientific Publications. Oxford, pp 71–114
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Golden, D.C., White, R.E., Tillman, R.W. et al. Partially acidulated reactive phosphate rock (PAPR) fertilizer and its reactions in soil. Fertilizer Research 28, 281–293 (1991). https://doi.org/10.1007/BF01054329
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01054329