Abstract
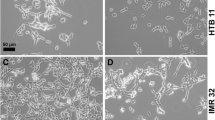
The experiments were carried out on the IMR-32 human neuroblastoma and NIE-115 murine neuroblastoma cultured cells. Peculiarities of the ion channel expression and their correlation with the main morphological parameters characterizing neuronal differentiation were studied under conditions of incubation of the cells with α2-interferon and 2′,5′-oligoadenylate, 2–5A. Twenty-four hours after addition of 1000 U act./ml interferon to the culture medium, a 56% increase in the mean projective surface of the IMR-32 cells was observed, and after a nine-day-long exposition this increase was 132%, as compared with the control. Mean total length of the cellular protrusions nearly doubled after nine-day-long incubation. Morphological and electrophysiological properties of the N1E-115 murine neuroblastoma cells showed practically no changes after incubation with human α2-interferon. Cultivation of the IMR-32 human neuroblastoma cells in the presence of 2–5A evoked insignificant changes in their morphological parameters. By contrast, the mean total length of neurites of the N1E-115 neuroblastoma protrusion-supplied cells increased more than a factor of five after eight-day-long incubation with 2–5A in a 1.0 µM concentration, and at a 0.01 µM 2–5A concentration this increase was about fourfold. At the same time, the projective surface exhibited no significant changes either in the neurite-supplied or the neurite-free cellular subpopulations. Twenty-four hours after the incubation with human α2-interferon had been begun, the density of sodium current in the IMR-32 human neuroblastoma cells increased by 250% compared with the control. A similar effect was observed after the addition of 2–5A to the medium: the density of sodium current approximately doubled. Cultivation of the neurite-supplied N1E-115 murine neuroblastoma cells was followed by a gradual increase in the density of fast sodium current both in control and 2–5A-influenced samples, but in the latter case this increase was significantly faster. In the neurite-free cells, the density of sodium current was 27% higher after their 24-h-long incubation with 2–5A, as compared with the control values (11.05±0.9 and 8.7±0.9 pA/pF, respectively). Longer incubation resulted in a sharp decrease in the density of potassium current. The results of our study are in agreement with the data about species-related individuality of α2-interferon and different intensity of its effects on the cells passing different stages of cellular differentiation.
Similar content being viewed by others
References
F. E. Ershov and A. S. Novokhatskii,Interferon and Its Inductors [in Russian], Meditsina, Moscow (1980).
B. Rossi Govanni, “Interferon and cell differentiation,”Interferon,6, 31–68 (1985).
A. G. Hovanessian, “Interferon-induced and double-stranded RNA-activated enzymes: a specific protein kinase and 2′,5′-oligoadenylate synthetase,”J. Interferon Res.,11, 199–205 (1991).
H. C. Schroder, R. J. Suhadolnik, W. Peleiderer, et al., “(2′–5′)-oligoadenylate and intracellular immunity against retrovirus infection,”Int. J. Biochem.,24, No.1, 55–63 (1992).
Ch. L. Schauf, “Ion channel diversity: a revolution in biology?,”Sci. Prog.,71, 459–478 (1987).
P. G. Kostyuk,Calcium Ions in Nerve Cell Function, Oxford Univ. Press, Oxford, New York, Tokyo (1992).
N. Kh. Pogorelaya, A. N. Savchenko, and Ya. M. Shuba, “A study of membrane calcium channels of pheochromocitoma cells in the course of their artificially induced differentiation,”Tsitologiya,31, No. 9, 1118 (1989).
N. Kh. Pogorelaya and A. F. Fomina, “An effect of pH shifts on the differentiation of cultivated neuroblastoma cells,”Neirofiziologiya,16, No. 842–845 (1984).
N. Toshio, T. Akinobu, and Y. Mitsunobu, “Characterization of two types of calcium channels in mouse neuroblastoma cells,”J. Physiol.,383, 231–251 (1987).
P. Kostyuk, N. Pronchuk, A. Savchenko, and A. Verkhratskii (Verkhratsky), “Calcium currents in aged rat neurons,”J. Physiol.,461, 467–483 (1993).
S. V. Vyatchenko-Karpinskii, N. Kh. Pogorelaya, I. S. Magura, et al., “Effects of α2-interferon on sodium channels in IMR-32 human neuroblastoma cells,”Biol. Membrany,9, No. 10/11, 1159–1160 (1992).
L. M. Pfeffer and Y. H. Tan, “Do second messengers play a role in interferon signal transduction,”Trends Biochem. Sci.,16, No. 9, 321–323 (1991).
J. D. Pollock, M. Krempin, and B. Rudy, “Differential effects of NRF, FGF, EGF, cAMP, and dexamethasone on neurite outgrowth and sodium channel expression in PC-12 cells,”J. Neurosci.,10, No.8, 2626–2637 (1990).
R. C. Berdan and J. C. Easaw, “Modulation of sprouting in organ culture after axotomy of an identified molluscan neuron,”J. Neurobiol.,23, No. 4, 433–450 (1992).
R. C. Berdan and R. L. Ridway, “Release of neurite outgrowth promoting factors byHelisoma central ganglia depends on neural activity,”Brain Res.,572, 132–138 (1992).
Author information
Authors and Affiliations
Additional information
Neirofiziologiya/Neurophysiology, Vol. 27, No. 3, pp. 199–207, May–June, 1995.
Rights and permissions
About this article
Cite this article
Vyatchenko-Karpinskii, S.V., Pogorelaya, N.K., Magura, I.S. et al. α2-Interferon- and oligoadenylate-induced morphological differentiation and modulation of the ion channels in neuroblastoma cells. Neurophysiology 27, 156–163 (1995). https://doi.org/10.1007/BF01053170
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01053170