Abstract
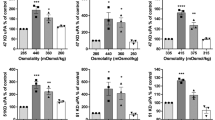
The function of proteases in brain tumor invasion is currently not well established. For tumors of epithelial and fibromatous origin collagenase production can enhance the invasive capacity of cells to penetrate basement membranes. We showed previously that a c-Ha-ras transformed glial cell line (CxT24neo3) invaded hamster brain tissuein vivo. These cells were also capable of invading reconstituted basement membrane and embryonic chick heartsin vitro. Since the histopathology of CxT24neo3 tumors mimics that of glioblastoma multiforme in humans, CxT24neo3 was used as the modelin vitro for this type of brain tumor. Presently, we detected by zymogram analysis a gelatinase that was secreted by CxT24neo3 and that had an apparent molecular mass of 62 kD.
To verify whether gelatinase affected invasionin vitro of these glial cells we determined the efficacy of a substrate specific collagenase inhibitor on invasionin vitro. GM6001 is a synthetic polypeptide that specifically occupies the substrate binding sites of metalloprotease. Since this drug did not show cytotoxicity, its specificity for metalloprotease is a valuable tool to evaluate the physiological function of these enzymes on invasion. We found that treatment of CxT24neo3 with GM6001 reduced the fraction of invading CxT24neo3 cells through reconstituted basement membrane. These data suggest that metalloproteases can stimulate brain tumor invasion.
Similar content being viewed by others
References
Johnson WH, Roberts NA, Borkakoti: Collagenase inhibitors: their design and potential therapeutic use. J Enzyme Inhibition 2:1–22, 1987
Mareel MM, Bracke ME, Boghaert ER: Tumor invasion and metastasis: major causes of therapeutic failure. Radiotherapy and Oncology 6:135–142, 1986
Mareel M, Bracke M, Bruyneel E, Van Larebeke N, De Mets M: Invasion and metastasis control: implications for increased therapeutic index of antitumor drugs. Cancer Treatment Rev 17: 335–338, 1990
Walsh JW, Zimmer SG, Oeltgen J, Markesbery WR: Invasiveness in primary intracranial tumors. Part 1. Neurosurgery 19:185–200, 1986
Laerum OD, Bjerkvig R, Steinsvag SK, de Ridder L: Invasiveness of primary brain tumors. Cancer and Metastasis Reviews 3: 223–236, 1984
Vaithilingam IS, Stroude EC, McDonald W, Del Maestro RF: General protease and collagenase (IV) activity in C6 astrocytoma cells, C6 spheroids and implanted C6 spheroids. Journal of Neuro-Oncology 10: 203–212, 1991
Taylor CM, Weiss JB, Lye RH: Raised levels of latent collagenase activating angiogenesis factor (ESAF) are present in actively growing human intracranial tumors. Br J Cancer 64: 164–168, 1991
Melchiori A, Albini A, Ray JM, Steuer-Stevenson WG: Inhibition of tumor cell invasion by a highly conserved peptide sequence from the matrix metalloprotease enzyme proseyment. Cancer Res 52: 2353–2356, 1992
Mareel MM: Invasion: therapeutic prospects. In: M. Mareel and K. Colman (Eds) Invasion. Oxford University Press, Oxford, New York, Tokoyo. 1984, pp 275–300, 1990
Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S: Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature (London) 284: 67–68, 1980
Nakatsukasa H: Type IV collagen — degrading enzyme activity in teratocellular carcinoma. Acta Med Okayama 40:83–91, 1986
Starkey JR: Cell-matrix interactions during tumor invasion. Cancer Metastasis Rev 9:113–123, 1990
Liotta LA, Stetler-Stevenson WG: Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res (suppl) 51: 5054s-5059s, 1991
Taylor CM, Thompson JM, Weiss JB: Matrix integrity and the control of angiogenesis. Int J Radiat Biol 60:61–64, 1991
Missirlis E, Karakiulakis G, Maragoudakis ME: Angiogenesis is associated with collagenons protein synthesis and degradiation in the chick chorioallantoic membrane. Tissue and Cell 22(4): 419–426, 1990
Grobelny D, Poncz L, Galardy RE: Inhibition of human skin fibroblast collagenase, thermolysin, and pseudomonas Aeruginosa elastase by peptide hydroxamic acids. Biochemistry 31: 7152–7154, 1992
Fetherston JD, Cotton J, Walsh J, Zimmer SG: Transformation of normal and transformed hamster cerebral cortex glial cells with activated c-Ha-ras results in the acquisition of a diffusely invasive phenotype. Oncogene research 5:25–30, 1989
Boghaert ER, Simpson JF, Zimmer SG: Invasionin vitro of malignant hamster brain tumor cells is influenced by the number of cells and the mode of malignant progression. Invasion Metastasis 12:12–23, 1992
Rasheed S, Nelson-Rees WA, Toth EM, Arnstein P, Gardner MB: Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer (Phila.) 33:1027–1033, 1974
Brown R, Marshall CJ, Pennie SB, Hall A: Mechanisms of activation of an N-ras gene in the human fibrosarcoma cell line Ht1080. EMBO J 3:1321–1326, 1984
Brown PD, Levy AT, Margulies IMK, Liotta LA, Stetler-Stevenson WG: Independent expression and cellular processing of M. 72,000 Type IV collagenase in human tumorigeneic cell lines. Cancer Research 50: 6181, 1990
Heussen C, Dowdle EB: Electrophoetic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulphate and copolymerized substrates. Anal Biochem 102:196–202, 1980
Mosmann T: Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63, 1983
Mareel M, Kint J, Meyvisch C: Methods of study of the invasion of C3H-mouse fibroblasts into embryonic chick heartin vitro. Virchows Arch (Cell Pathol) 30: 95–111, 1977
Boghaert ER, Simpson J, Jacob RJ, Lacey T, Walsh JW, Zimmer SG: The effect of dibutyryl cAMP on morphological differentiation growth and invasionin vitro of a hamster brain tumor cell line: A comparative study of dBcAMP effects in 2- and 3-dimensional cultures. Int J Cancer 47: 610–618, 1991
Attia MA, Weiss DW: Immunology of spontaneous mammary carcinomas in mice. V. Acquired tumor resistance and enhancement in strain-A mice injected with mammary tumor virus. Cancer Res 26:1787–1800, 1966
Bracke ME, Van Cauwenberghe RM-L, Mareel MM: (+)-catechin inhibits the invasion of malignant fibrosarcoma cells into chick heartin vitro. Clin Exp Metastasis 2:161–170, 1984
Reich R, Thompson EW, Iwamoto Y, Martin GR, Deason JR, Fuller GC, Miskin R: Effects of inhibitors of plasminogen activator, serine proteinases and collagenase IV on the invasion of basement membranes by metastatic cells. Cancer Research 48: 3307–3312, 1988
de Ridder L, Calliauw L: Invasion of human brain tumorsin vitro: relationship to clinical evolution. J Neurosurg 72:589–593, 1990
Zucker S: A critical appraisal of the role of proteolytic enzymes in cancer invasion: emphasis on tumor surface proteases. Cancer Investigation 6(2): 219–231, 1988
Mignatti P, Robbins E, Rifkin DB: Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell 47: 487–498, 1986
Paganetti PP, Coroni P, Schwab ME: Glioblastoma infiltration into central nervous system tissuein vitro: involvement of a metalloprotease. J Cell Biol 107: 2281–2291, 1988
Murphy P, Hart DA: Modulation of plasminogen activator and plasminogen activator inhibitor expression in the human U373 glioblastoma/astrocytoma cell line by inflammatory mediators. Exptl Cell Research 198: 93–100, 1992
Wiestier OD, Aguzzi A, Schneeman M, Eibl R, von Deimling A, Kleih P: Oncogene complementation in fetal brain transplants. Cancer Res 52: 3760–3767, 1992
Westphal M, Herrmann HD: Growth factor biology and oncogene activation in human gliomas and their implication for specific therapeutic concepts. Neurosurgery 25:681–694, 1989
Dietrich V, Eckermann O, Schmidtke J: Rare Ha-ras and cmos alleles in patients with intracranial tumors. Neurosurgery 38: 587–589, 1988
Gerosa MA, Talarico D, Fognani C, Raimondi E, Colombatti M, Tridente G, DeCarli L, Delia Valle G: Overexpression of Ki-ras oncogene and epidermal growth factor receptor gene in human glioblastomas. J Natl Cancer Inst 81: 63–67, 1989
Arvanitis D, Malliri A, Antoniou D, Linardopoulos S, Field JK, Spandidos DA: Ras p21 expression in bain tumors: elevated expression in malignant astrocytomas and glioblastomas multiforme.In vivo 5: 317–322, 1991
Libermann TA, Razon M, Bartal AD, Yarden Y, Schlesigner J, Soreq H: Expression of epidermal growth factor receptors in human brain tumors. Cancer Res 44: 753–760, 1984
Garbisa S, Pozatti R, Muschel RJ, Saffiotti U, Ballin M, Goldfarb RH, Khoury G, Liotta L: Secretion of type IV collagenolytic protease and metastatic phenotype: induction by transfection with c-Ha-ras but not c-Ha-ras plus Ad2-Ela. Cancer Res 47:1523–1528, 1987
Rutka JT, Apodaca G, Stern R, Rosenblum M: The extracellular matrix of the central and peripheral nervous system: structure and function. J Neurosurg 69:155–170, 1988
Bjerkvig R, Laerum OD, Rucklidge GJ: Immunocytochemical characterization of extracellular matrix proteins expressed by cultured glioma cells. Cancer Res 49:5424–5428, 1989
McKeever PE, Fligiel SEG, Varani J, Castle RL, Hood TW: Products of cells cultured from gliomas. VII. Extracellular matrix proteins of gliomas which contain glial fibrillary acidic protein. Laboratory Investigation 60: 286–295, 1986
Eccleston PA, Mirsky R, Jessen KR: Type 1 collagen preparations inhibit DNA synthesis in glial cells of the peripheral nervous system. Experimental Cell Research 182:173–185, 1989
Alvord EC: Why do gliomas not metastasize? Arch Neurol 33: 73–75, 1976
Vaithilingam IS, McDonald W, Stromb EC, Cook RA, Del Maestro RF: Proteolytic activity during the growth of C6 astrocytoma in the murine spheroid implantation model. Can J Neurol Sci 19:17–22, 1992
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boghaert, E.R., Chan, S.K., Zimmer, C. et al. Inhibition of collagenolytic activity relates to quantitative reduction of invasionin vitro in a c-Ha-ras transfected glial cell line. J Neuro-Oncol 21, 141–150 (1994). https://doi.org/10.1007/BF01052898
Issue Date:
DOI: https://doi.org/10.1007/BF01052898