Summary
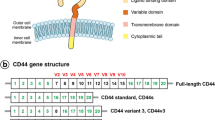
CD44s (standard form of CD44) is a transmembrane glycoprotein whose external domain displays extracellular matrix adhesion properties by binding both hyaluronic acid (HA) and collagen. The cytoplasmic domain of CD44s interacts with the cytoskeleton by binding directly to ankyrin. It has been shown that post-translational modifications, such as phosphorylation (by protein kinase C), acylation (by acyl-transferase) and GTP-binding enhance CD44's interaction with cytoskeletal proteins. Most importantly, the interaction between CD44s and the cytoskeletal protein, ankyrin, is required for the modulation of CD44s cell surface expression and its adhesion function.
Recently, a number of tumor cells and tissues have been shown to express CD44 variant (CD44v) isoforms. Using RT-PCR and DNA sequence analyses, we have found that unique CD44 splice variant isoforms are expressed in both prostate and breast cancer cell lines and carcinomas. Most importantly, intracellular ankyrin is preferentially accumulated underneath the patched/capped structures of CD44 variant isoform in both breast and prostate cancer cells attached to HA-coated plates. We propose that selective expression of CD44v isoforms unique for certain metastatic carcinomas and their interaction with the cytoskeleton may play a pivotal role in regulating tumor cell behavior during tumor development and metastasis.
Similar content being viewed by others
References
Stamper HB Jr, Woodruff JJ: Lymphocyte homing into lymph nodes:in vitro demonstration of the selective affinity of recirculating lymphocytes for high endothelial venules. J Exp Med 144: 828–833, 1976
Jalkanen S, Steere AC, Fox RI, Butcher EC: A distinct endothelial recognition system that controls lymphocyte traffic into inflamed synovium. Science 233: 556–558, 1986
Berg EL, Goldstein LA, Jutila MA, Nakache M, Picker LJ, Streeter PR, Wu NW, Zhou D, Butcher EC: Homing receptors and vascular addressins: cell adhesion molecules that direct lymphocyte traffic. Immunol Rev 108: 5–18, 1989
Jalkanen S, Bargatze RF, de los Toyos J, Butcher EC: Lymphocyte recognition of high endothelium antibodies to distinct epitopes of an 85–95 kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol 105: 983–990, 1987
Goldstein LA, Zhou DF, Picker LJ, Minty CN, Bargatze RF, Ding JF, Butcher EC: A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell 56:1063–1072, 1989
Haynes BF, Telen MJ, Hale LP, Denning SM: CD44-a molecule involved in leukocyte adherence and T-cell activation. Immunol Today 10: 423–427, 1989
Miyake K, Medina KL, Hayashi SI, Ono S, Hamaoka T, Kincade PW: Monoclonal antibodies to Pgp-1/CD44 block lympho-hemopoiesis in long-term bone marrow culture. J Exp Med 171: 477–488, 1990
Trowbridge IS, Lesley J, Sculte R, Hyman R, Trotter J: Biochemical characterization and cellular distribution of a polymorphic murine cell-surface glycoprotein expressed on lymphoid tissues. Immunogenetics 15: 299–312, 1982
Lesley J, Trowbridge IS: Genetic characterization of a murine polymorphic cell surface glycoprotein. Immunogenetics 15: 313–320, 1982
Kalomiris EL, Bourguignon LYW: Mouse T-lymphoma cells contain a transmembrane glycoprotein (GP85) which binds ankyrin. J Cell Biol 106: 319–327, 1988
Kalomiris EL, Bourguignon LYW: Lymphoma protein kinase C is associated with the transmembrane glycoprotein, GP85 and may function in GP85-ankyrin binding. J Biol Chem 264: 8113–8119, 1989
Bourguignon LYW, Kalomiris E, Lokeshwar VB: Acylation of the lymphoma transmembrane glycoprotein, GP85 may be required for GP85-ankyrin interaction. J Biol Chem 266: 11761–11765, 1991
Lokeshwar VB, Bourguignon LYW: Post-translational protein modification and expression of ankyrin-binding site(s) in GP85 (Pgp-1.CD44) and its biosynthesis precursors during T-lymphoma membrane biosynthesis. J Biol Chem 266: 17983–17989, 1991
Lesley J, Hyman R, Schulte R: Evidence that the Pgp-1 glycoprotein is expressed on thymus-homing progenitor cells of the thymus. Cell Immunol 91: 397–403, 1985
Lesley J, Trotter J, Hyman R: The Pgp-1 antigen is expressed on early fetal thymocytes. Immunogenetics 22: 149–157, 1985
Shimizu Y, Shaw S: Lymphocyte interactions with extracellular matrix. FASEB J 5: 2292–2299, 1991
Zhou DFH, Ding JF, Picker LJ, Bargatze RF, Butcher EC, Goeddel DV: Molecular cloning and expression of Pgp-1: the mouse homolog of the human H-CAM (Hermes) lymphocyte homing receptor. J Immunol 143: 3390–3395, 1989
Wolffe E, Gause WC, Pelfrey CM, Holland SM, Steinberg AD, August JT: The cDNA sequence of mouse Pgp-1 and homolog to human CD44 cell surface antigen and proteolycan core/link protein. J Biol Chem 265: 341–347, 1990
Underhill C: CD44: The hyaluronan receptor. J Cell Sci 103: 293–298, 1992
Lesley J, Schulte R, Hyman R: Binding of hyaluronic acid to lymphoid cell lines is inhibited by monoclonal antibodies against Pgp-1. Exp Cell Res 187: 224–233, 1990
Culty M, Miyake K, Kincade PW, Silorski E, Butcher EC, Underhill C: The hyaluronate receptor is a member of the CD44(H-CAM) family of cell surface glycoproteins. J Cell Biol 111: 2765–2774, 1990
He Q, Lesley J, Hyman R, Ishihara K, Kincade PW: Molecular isoforms of murine CD44 and evidence that the membrane proximal domain is not critical for hyaluronate recognition. J Cell Biol 119: 1711–1719, 1992
Carter WG, Wayner EA: Characterization of the class III collagen receptor, a phosphorylated transmembrane glycoprotein expressed in nucleated human cells. J Biol Chem 263: 4193–4201, 1988
Bourguignon LYW, Lokeshwar VB, Chen X, Kerrick WGL: Hyaluronic acid-induced lymphocyte signal transduction and HA receptor (GP85-CD44)-cytoskeleton interaction. J Immunol 151: 6634–6644, 1993
Lokeshwar VB, Bourguignon LYW: The lymphoma transmembrane glycoprotein GP85(CD44) is a novel G-protein which regulates GP85(CD44)-ankyrin interaction. J Biol Chem 267: 22073–22078, 1992
Lokeshwar VB, Fregien N, Bourguignon LYW: Ankyrin binding domain of CD44(GP85) is required for the expression of hyaluronic acid-mediated adhesion function. J Cell Bio 1126:1099–1109, 1994
Matsumura Y, Tarin D: Significance of CD44 gene products for cancer diagnosis and disease evaluation. Lancet 340: 1053–1058, 1992
Heider KH, Hofmann M, Hors E, Berg FVD, Ponta H, Herrlich P, Pals ST: A human homologue of the rat metastasis associated variant of CD44 is expressed in colorectal carcinomas and adenomatous polyps. J Cell Biol 120: 227–233, 1993
Tanabe KK, Ellis LM, Sayer H: Expression of CD44R1 adhesion molecule in colon carcinomas and metastasis. Lancet 341: 725–726, 1993
Gunthert U, Hoffman M, Rudy W, Reber S, Zoller M, Haűmann I, Matzku S, Wenzel A, Ponta H, Herrlich P: A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 65: 13–24, 1991
Rudy W, Hofmann M, Schwartz-Albier R, Zoller M, Heider KH, Ponta H: The two major CD44 proteins expressed on a metastatic rat tumor cell line are derived from different splice variants: Each are individually suffices to confer metastatic behavior. Cancer Res 53: 1262–1268, 1993
Arch R, Wirth K, Hofmann M, Ponta H, Matzku S, Herrlich P, Zoller M: Participation in normal immune responses of a metastasis-inducing splicing variant of CD44. Science 257: 682–685, 1992
Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth V, Bell JI: Genomic structure of DNA coding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci USA 89: 12160–12164, 1992
Tolg C, Hofmann M, Herrlich P, Ponta H: Splicing choice from ten variant exons establishes CD44 variability. Nucleic Acids Res 21: 1225–1229, 1993
Iida N, Bourguignon LYW: New CD44 splice variant associated with human breast cancers. J Cell Physiol 162: 127–133, 1995
Lokeshwar VB, Bourguignon LYW: Hyaluronic acid receptor GPU6, is a new CD44 variant in endothelial cells. Mol Biol Cell 4: 177a, 1993
Dougherty GJ, Lansdorp PM, Cooper DL, Humphries RK: Molecular cloning of CD44R1 and CD44R2, two novel isoforms of human CD44 lymphocyte homing receptor expressed by hemopoietic cells. J Exp Med 174: 1–5, 1991
Hofmann M, Rudy W, Zoller M, Tolg C, Ponta H, Herrlich P, Gunthert U: CD44 splice variants confer metastatic behavior in rats: homologous sequences are expressed in human tumor cell lines. Cancer Res 51: 5292–5297, 1991
Salmi M, Kristiina GV, Sointu P, Grenman R, Kalimo H, Jalfnkanen S: Regulated expression of exon v6 containing isoforms of CD44 in man: Downregulation during malignant transformation of tumors of squamocellular origin. J Cell Biol 122: 431–442, 1993
Brothman AR, Lesho LJ, Somers KD, Wright GL Jr, Merchant DJ: Phenotypic and cytogenetic characterization of a cell line derived from primary prostate carcinoma. Int J Cancer 44: 898–903, 1989
Loop SM, Rozanski TA, Ostenson RC: Human primary prostate tumor cell line, ALVA-31: a new model for studying the hormonal regulation of prostate tumor cell growth. Prostate 22: 93–108, 1993
Dumont P, Petein M, Lespagnard L, Tueni E, Coune A: Unusual behavior of the LNCaP prostate tumor xenografted in nude mice.In Vivo: 167–170, 1993
Horst E, Meijer CJLM, Radaszkiewicz T, Ossekoppele GJ, Van Krieken JHJM, Pals ST: Adhesion molecules in the prognosis of diffuse large-cell lymphoma: expression of a lymphocyte homing receptor (CD44), LFA-1 (CD11a/1b), and ICAM/(CD54). Leukemia 4: 595–599, 1990
Jalkanen S, Joensuu H, Oderstr S, Ko O, Klemi P: Lymphocyte homing and clinical behavior of non-Hodgkin's lymphoma. J Clin Invest 87: 1835–1840, 1991
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bourguignon, L.Y.W., Iida, N., Welsh, C.F. et al. Involvement of CD44 and its variant isoforms in membrane-cytoskeleton interaction, cell adhesion and tumor metastasis. J Neuro-Oncol 26, 201–208 (1995). https://doi.org/10.1007/BF01052623
Issue Date:
DOI: https://doi.org/10.1007/BF01052623