Abstract
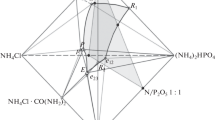
Data from four solubility studies for liquid fertilizer solutions containing three or more plant nutrients are presented on equilateral triangles. Isoconcentration contour lines indicate the maximum solubility of the nutrients as the sum of the plant food components, i.e., total plant nutrient = %N + %P2O5 + %K2O.
One study was based on an ammoniated phosphoric acid (80% polyphosphate level)-urea-potassium chloride-water system. The base solution was a nominal 11-37-0 grade, with one-fifth of the total phosphate derived from wet-process phosphoric acid and the remainder from electric-furnace acid. Two of the studies were with nonchloride sources of potash, and the fourth study was with ammonium sulfate as a source of sulfur and supplemental nitrogen.
All solubility data were measured at 0°C. However, estimates of solubility at other temperatures can be made by proper use of temperature-solubility factors. Also given are areas on the figures indicating precipitating salts, as determined by petrographic examinations.
Similar content being viewed by others
References
Douglas JR and Tisdale SL (1971) The ammonium sulfate situation. The Sulfur Institute Monograph No. 1, Washington, D.C.: The Sulfur Institute
Gray RC (1977) Foliar fertilisation with primary nutrients during the reproductive stage of plant growth. Proceedings No. 164, London: The Fertilizer Society of London
Kapusta EC (1958) Potash in liquid fertilizer manufacture. Commer Fert 97: 24–29
Linke WF, ed. (1958) Solubilities: inorganic and metal organic compounds, 4th ed., Washington, D.C.: American Chemical Society
Scott WC, Wilbanks JA and Faulkner LC (1976) Indicators show continued growth in use of liquid fertilizers. Fert Solutions 20: 54, 56, 58–60, 62, 64
Silcock HL, ed. (1979) Solubilities of inorganic and organic compounds. New York: Pergamon Press
Slack AV, Hatfield JD, Shaffer HB and Driskell JC (1959) Solubility relationships in liquid mixed fertilizer systems. J Agric Food Chem 7: 404–408
Slack AV, Potts JM and Shaffer HB (1964) Solubility relationships in liquid fertilizer systems based on superphosphoric acid. J Agric Food Chem 12: 154–157
Slack AV, Potts JM and Shaffer HB (1965) Effect of polyphosphate content on properties and use of liquid fertilizers. J Agric Food Chem 13: 165–171
Tinker PB, Reed L, Legg C and Hojer-Pederson S (1977) The effects of chloride in fertiliser salts on crop seed germination. J. Sci Fd Agric 28: 1045–1051
Tisdale SL and Nelson WLN (1975) Soil fertility and fertilizers, 3rd ed. New York: Macmillan
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shaffer, H.B. Solubility studies in multicomponent fertilizer solutions. Fertilizer Research 15, 89–99 (1988). https://doi.org/10.1007/BF01049190
Issue Date:
DOI: https://doi.org/10.1007/BF01049190