Abstract
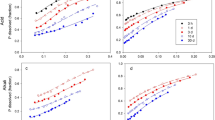
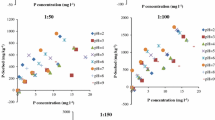
The influence of soil pH on the dissolution of phosphate rock fertilizers was investigated in laboratory experiments with reactive North Carolina phosphate rock (PR) in a lateritic soil adjusted to several pH values. Increased soil pH resulted in decreased dissolution as estimated by the increase in exchangeable calcium (ΔCa) method. The extent of PR dissolution was related to soil pH by an equation of the form Log ΔCa = a–b pH, and it increased with contact period and rate of PR application. Increased plant available P, as estimated by NaHCO3 soluble-P (ΔBicP) was about one third of the P dissolved from PR. ΔBicP was related to soil pH by an equation of the form Log ΔBic P = c–d pH. Dissolution of PR in soil can be considered as a simple chemical reaction between apatite and hydrogen ions supplied by soil constituents.
Similar content being viewed by others
References
Official Methods of Analysis (1975) 12th edn., AOAC, Washington D.C.
Barnes JS and Kamprath EJ (1975) Availability of North Carolina rock phosphate applied to soils. NC Agric Exp Stn Tech Bull no. 229
Barrow NJ and Shaw TC (1976) Sodium bicarbonate as an extractant for soil phosphate, 1. Separation of the factors affecting the amount of secondary adsorption, Geoderma 16:91–107
Chien SH, Clayton WR and McClellan GH (1980) Kinetics of dissolution of phosphate rocks in soils. Soil Sci Soc Am J 44:260–264
Chu CR, Moschler WW and Thomas GW (1962) Rock phosphate transformations in acid soils. Soil Sci Soc Am Proc 26:476–478
Ellis R Jr, Quader MA and Truog E (1955) Rock phosphate availability as influenced by soil pH. Soil Sci Soc Am Proc 19:484–487
Graham ER (1955) Availability of natural phosphates according to energy changes. Soil Sci Soc Am Proc 19:26–29
Hughes JC and Gilkes RJ (1984) Effect of chemical extractants on the estimation of rock phosphate fertilizer dissolution. Aust J Soil Res 22:475–481
Jones US (1948) Availability of phosphorus in rock phosphate as influenced by potassium and nitrate salts, lime and organic matter. J Am Soc Agron 40:765–770
Joos LL and Black CA (1950) Availability of phosphate rock as affected by particle size and contact with Bentonite and soil of different pH values. Soil Sci Soc Am Proc 15:69–75
Murphy J and Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Olsen SR, Cole CV, Watanabe FS and Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular 939, U.S. Government Printing Office, Washington, DC
Parfitt RL (1979) The availability of P from phosphate-goethite bridging complexes. Desorption and uptake by rye grass. Plant and Soil 53:55–65
Peaslee DE, Anderson CA, Burns GR and Black CA (1962) Estimation of relative value of phosphate rock and superphosphate to plants on different soils. Soil Sci Soc Am Proc 26:566–570
Smyth TJ and Sanchez PA (1982) Phosphate Rock dissolution and availability in Cerrado soils as affected by phosphorus sorption capacity. Soil Sci Soc Am J 46:339–345
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kanabo, I.A.K., Gilkes, R.J. The role of soil pH in the dissolution of phosphate rock fertilizers. Fertilizer Research 12, 165–173 (1987). https://doi.org/10.1007/BF01048916
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01048916