Abstract
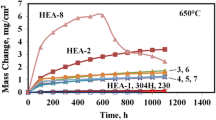
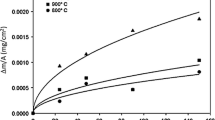
In continuation of previous work, the oxidation behavior of three more alloys, namely UHBA 25L, Sanicro 28, and Inconel 690, was studied at high temperatures (600 to 1200°C). The oxidation kinetics of UHBA 25L and Sanicro 28 followed the parabolic-rate law at 800 and 1000°C. At 600°C oxidation rates were very low, while at 1200° C the parabolic-rate law was initially observed, followed by “breakaway.” Both alloys suffered extensive spalling at all temperatures, despite their high Cr contents of about 25% and 27%, respectively. This is atrributed to significant amounts of Mn present. Sanicro 28, with higher Cr, suffered an early breakaway at 1200°C because of the presence of Mo, which forms a low-melting oxide. Inconel 690 showed mixed behavior at 600 and 800°C, parabolic at 1000°C and cubic at 1200°C. Inconel 690 and Incoloy 800H gave the best overall performance for the range of temperature and exposure time under study. This may be ascribed to the absence of Mo, the presence of small amounts of Ti and Al, and relatively small amount of Mn in these alloys.
Similar content being viewed by others
References
N. Hussain, K. A. Shahid, I. H. Khan, and S. Rahman,Oxid. Met. 41, 253 (1994).
G. Rundel and J. McConnel,Oxid. Met. 36, 253 (1991).
N. Hussain, G. Schanz, S. Leistikow, and K. A. Shahid,Oxid. Met. 22, 405 (1989).
A. L. Marasco and D. J. Young,Oxid. Met. 36, 157 (1991).
D. L. Douglass and F. Rizzo-Assuncao,Oxid. Met. 29, 272 (1988).
F. Gesmundo, C. de Asmundis, G. Battilana, and E. Ruedl,Werkst. Korros. 38, 368 (1987).
F. H. Stott, F. I. Wei, and C. A. Enahoro,Werkst. Korros. 40, 198 (1989).
D. Caplan, P. E. Beaubien, and M. Cohen,Trans. AIME. 233, 766 (1965).
D. L. Douglass, F. Gesmundo, and C. de Asmundis,Oxid. Met. 25, 235 (1986).
K. R. Peters, D. P. Whittle, and J. Stringer,Corros. Sci. 16, 791 (1976).
M. H. LaChance and R. I. Jaffee,Trans. A.S.M. 48, 595 (1956).
S. S. Brenner,J. electrochem. Soc. 102, 16 (1955).
D. Caplan and M. Cohen,Trans. AIME. 4, 1057 (1954).
S. Leistikow,Basic Principles and Forms of High Temperature Corrosion, VDI-Report No. 235, 1975, pp. 125–143.
Y. Shida and T. Moroishi,Oxid. Met. 37, 327 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hussain, N., Shahid, K.A., Khan, I.H. et al. Oxidation of high-temperature alloys (superalloys) at elevated temperatures in air. II. Oxid Met 43, 363–378 (1995). https://doi.org/10.1007/BF01047036
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01047036