Abstract
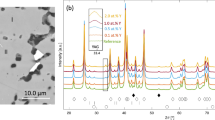
Yttrium-coated Ni3Al with post heat treatment has shown good high-temperature oxidation resistance. To understand the effect of the Y-coating and post heat treatment on the oxidation resistance of Ni3Al, the specimens were coated with Y by an ion-plating method, and heat treatment was performed at low oxygen level before or after the Y-coating was applied. Performance of the Y-coated Ni3Al was evaluated by isothermal and cyclic oxidation tests. A simple deposition of Y on Ni3Al did not change the oxidation kinetics, but the post heat treatment after Y-ion plating significantly decreased the oxidation rate of Ni3Al. The scale formed on Y-coated Ni3Al with post heat treatment after Y-ion plating showed a fine and dense structure which was grain refined by the presence of a (Y, Al) O-type oxide in the scale. The coated Y layer becomes a Y-Al compound during heat treatment. The presence of the (Y, Al)O-type oxide in grain boundaries or the lattice of Al2O3 modify the diffusion rate of Al and oxygen, and the oxide microstructure during oxidation. Improvement of cyclic-oxidation resistance of Ni3Al by the Y-coating occurs because the presence of (Y, Al)O-type oxide develops fine-grain oxides which can easily relieve the growth stress.
Similar content being viewed by others
References
K. Aoki and O. Izumi,J. Jpn. Inst. Metal 43, 1190 (1979).
E. Lang,The Role of Active Elements in the Oxidation Behavior of High Temperature Metals and Alloys (Elsevier, London, 1989).
T. A. Ramanarayanan, M. Raghavan, and R. Petkovic-Luton,J. Electrochem. Soc. 131, 923 (1984).
F. A. Golightly, F. H. Stott, and G. C. Wood,J. Electrochem. Soc. 126, 1035 (1979).
J. D. Kuenzly and D. L. Douglass,Oxid. Met. 8, 139 (1974).
K. Y. Kim, S. H. Kim, K. W. Kwon, and I. H. Kim,Oxid. Met. 41, 179 (1994).
F. S. Pettit,Trans. Metall. Soc. AIME 239, 1296 (1967).
A. Steiner and K. L. Komarek,Trans. Metall. Soc. AIME 230, 786 (1964).
I. Barin,Thermochemical Data of Pure Substances (VCH, New York, 1989).
I. A. Kvernes,Oxid. Met. 6, 45 (1994).
G. M. Ecer, R. B. Singh, and G. H. Meier,Oxid. Met. 18, 55 (1982).
P. Y. Hou and J. Stringer,J. Mater. Sci. Eng. 87, 295 (1987).
Y. Saito, T. Maruyama, and T. Amano,J. Mater. Sci. Eng. 87, 279 (1987).
M. M. El-Aiat and F. A. Kröeger,J. Am. Ceram. Soc. 63, 280 (1980).
M. Landkok, A. V. Levy, D. H. Boone, R. Gray, and E. Yanity,Corrosion 41, 344 (1985).
J. Stringer, B. A. Wilcox, and R. I. Jaffee,Oxid. Met. 5, 11 (1972).
R. M. Canon and R. L. Coble, inDeformation of Ceramic Materials, R. C. Bradt and R. E. Tressler, eds. (Plenum Press, New York, 1975).
K. Y. Kim, J. H. Jun, and H. G. Jung,Oxid. Met. 40, 321 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jung, H.G., Kim, K.Y. Effect of yttrium coating on the oxidation behavior of Ni3Al. Oxid Met 46, 147–167 (1996). https://doi.org/10.1007/BF01046888
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01046888