Abstract
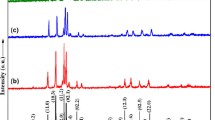
The system TiC−HfC−“MoC” was investigated by means of melting point, differentiothermoanalytical, X-ray diffraction and metallographic techniques on hotpressed as well as melted alloy specimens. A constitutional diagram from 1500°C through the melting range was established.
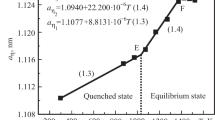
Investigation of the (Hf, Mo)C system (isopleth: HfC0.98−“MoC1.0”) showed a small miscibility gap within the cubic monocarbide solution (δ) [Tc=1630°C, (HfC)0.45(MoC)0.55]. The miscibility gap interacts with the solvus curve with a monotectoid-like decomposition reaction at 1575°C, (HfC)≈0.35(MoC)≈0.65.At temperatures below 1630°C, phase equilibria within TiC−HfC−“MoC” are characterized by a large miscibility gap connecting the TiC−HfC and HfC−MoC boundary systems. Additions of “MoC” to TiC−HfC alloys decrease the critical temperature (1780°C); additions of TiC to HfC−“MoC” alloys raise the critical temperature (1630°C). No maximum type ternary critical point or saddle point was found to occur.
Isothermal sections were prepared at 1500°C and 1650°C. At temperatures above 1960°C (μ-MoC+C⇄δ-MoC) a complete solid solution (δ-B 1) is formed within TiC−HfC−“MoC”. The melting behaviour (liquidus projection of TiC−HfC−“MoC”) shows flat melting temperatures in the “MoC” corner but extremely heterogeneous melting near the TiC−HfC boundary.
Isothermal sections have been calculated assuming regular solutions.
Similar content being viewed by others
References
E. Rudy, J. Less Common Metals33, 43 (1973).
E. Rudy andJ. Throop, Mh. Chem.104, 1164 (1973).
P. Rogl, S. Naik, andE. Rudy, Mh. Chem.108, 1189, 1213 (1977).
E. Rudy, Compendium of Phase Diagram Data, AFML-TR-65-2, V, 1969.
V. N. Eremenko, T. Ya. Velikanova, andS. V. Shabanova, in: Refractory Carbides (G. V. Samsonov, Hrsg.) English Translation. New York-London: Consultants Bureau. 1974.
O. I. Shulishova andI. A. Shcherbak, Izv. Akad. Nauk SSSR Neorgan. Mater.2, 2145 (1966).
W. N. Eremenko, T. Ya. Velikanova, S. W. Shabanova, andL. W. Artjuch, Colloq. Internat. CNRS205, 277 (1972).
E. Rudy, unpublished results.
E. Parthé andK. Yvon, Acta Cryst.B 25, 153 (1970).
H. Nowotny, R. Kieffer, F. Benesovsky, C. E. Brukl, andE. Rudy, Mh. Chem.90, 669 (1959).
P. Rogl, to be published.
J. Prigogine andR. Defay, Chem. Thermodynamik. Leipzig: 1962.
J. Meijering, The Physical Chemistry of Metallic Solutions and Intermetallic Compounds, Nat. Phys. Sympos. London: HMSO. 1958.
Author information
Authors and Affiliations
Additional information
With 5 Figures
Rights and permissions
About this article
Cite this article
Rogl, P., Naik, S.K. & Rudy, E. A constitutional diagram of the system TiC−HfC−“MoC”. Monatshefte für Chemie 108, 1325–1337 (1977). https://doi.org/10.1007/BF01046446
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01046446