Summary
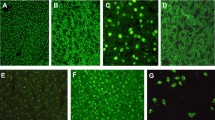
Four serum proteinase inhibitors, α1-macroglobulin, α1-proteinase inhibitor, α1-inhibitor3 and α1-acute phase macroglobulin, were localized in rat liver by immunofluorescent techniques. α1-Macroglobulin was observed predominantly in the sinusoids and α1-inhibitor3 in hepatocytes. In contrast, α1-proteinase inhibitor was localized in two sites, sinusoids and parenchymal cells. The fourth inhibitor tested, α2-acute phase macroglobulin, was barely detectable in normal liver. However, some appeared to be present in the extrahepatocyte space.
Similar content being viewed by others
References
Ashwell, G. &Morell, A. G. (1974) The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins.Adv. Enzym. 41, 99–129.
Barrett, A. J. &Starkey, P. M. (1973) The interaction of α2-macroglobulin with proteinases. Characterization and specificity of the reaction and hypothesis concerning its molecular mechanism.Biochem. J. 133, 709–24.
Cassiman, J. J., Van Leuven, F., Van Der Schueren, B. &Van Den Berghe, H. (1980) Immunohistochemical localization of human α2 macroglobulin macroglobulin in connective tissue.Cell Tiss. Res. 213, 301–10.
Coons, A. H. (1961) The beginnings of immunofluorescence.J. Immun. 87, 499–503.
DeBanne, M. T., Bell, R. &Dolovich, J. (1975) Uptake of proteinase-macroglobulin complexes by macrophages.Biochim. biophys. Acta 411, 295–304.
Esnard, F. & Gauthier, F. (1980) Purification and physiochemical characterization of a new rat pancreatic elastase and normal and inflammatory rat serum.Biochim. biophys. Acta 526.
Gan, J. C. (1979) Catabolism of desialylated human plasma α1 antitrypsin and its trypsin complex in the rat.Archs Biochem. Biophys. 194, 149–56.
Gauthier, F., Genell, S., Mouray, H. &Ohlsson, K. (1978). Thein vitro interactions of rat pancreatic elastase and normal and inflammatory rat serum.Biochim. biophys. Acta 526, 218–26.
Gauthier, F., Genell, S., Mouray, H. &Ohlsson, K. (1979) Interactionsin vitro andin vivo between rat serum protease inhibitors and anodal and cathodal trypsin and chymotrypsin.Biochim. biophys. Acta 566, 200–10.
Gauthier, F., Gutman, N., Muh, J. P. &Mouray, H. (1976) Isolation of two rat α-1-macroglobulin components by means of preparative isotachophoresis.Analyt. Biochem. 71, 181–5.
Gauthier, F. &Mouray, H. (1976) Rat α2 acute phase macroglobulin. Isolation and physiochemical properties.Biochem. J. 159, 661–5.
Gauthier, F. &Ohlsson, K. (1978) Isolation and some properties of a new enzyme binding protein in rat plasma.Hoppe-Seyler's Z. Physiol. Chem. 359, 987–92.
Heimburger, N. (1975) Proteinase inhibitors of human plasma their properties and control functions. InProteases and Biological Control. (edited byReich, E., Rifkin, D. B. andShaw, E.),Cold Spring Harbor Conferences on Cell Proliferation, Vol. 2, pp. 367–386.
Hovi, T., Mosher, D. &Vaheri, A. (1977) Cultured human monocytes synthesize and secrete α2-macroglobulin.J. exp. Med. 145, 1580–9.
Laskowski, M., Jr &Kato, I. (1980) Protein inhibitors of proteinases.Ann. Rev. Biochem. 49, 593–626.
Maurice, M., Feldmann, G., Druet, P., Laliberte, F. &Bouige, D. (1979) Immunoperoxidase localization of albumin in hepatocytes of nephrotic rats with special reference to changes in the Golgi apparatus.Lab. Invest. 40, 39–45.
Maxfield, F. R., Willingham, M. C., Haigler, H. T., Dragsten, P. &Pastan, I. H. (1981) Binding, surface mobility, internalization, and degradation of Rhodamine-labeled α2-macroglobulin.Biochemistry 20, 5353–8.
Mosher, D. F., Saksela, O. &Vajeri, A. (1977) Synthesis and secretion of alpha-2-macroglobulin by cultured adherent lung cells.J. clin. Invest. 60, 1036–45.
Ohlsson, K. (1971) Elimination of125I-Trypsin α-macroglobulin complexes from blood by reticuloendothelial cells in dog.Acta physiol. scand. 81, 269–72.
Petrusz, P., Ordronneau, P. &Finley, J. C. W. (1980) Criteria of reliability for light microscopic immunocytochemical staining.Histochem. J. 12, 333–48.
Stauber, W. T. &Ong, S. H. (1981) Extravascular localization of albumin and IgG in normal tonic and phasic muscles of the rat.Histochem. J. 13, 1009–15.
Turner, B. M. &Turner, V. S. (1980) Secretion of α1-antitrypsin by an established human hepatoma cell line and by human/mouse hybrids.Somatic Cell Genetics 6, 1–14.
Van Leuven, F., Cassiman, J. J. &Van Den Berghe, H. (1978) Uptake and degradation of α2-macroglobulin protease complexes in human cells in culture.Expl Cell. Res. 117, 273–82.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stauber, W., Fritz, V., Esnard, F. et al. Immunofluorescent localization of four serum proteinase inhibitors in normal rat liver. Histochem J 15, 161–166 (1983). https://doi.org/10.1007/BF01042284
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01042284