Summary
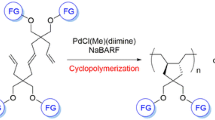
Cyclopolymerization was carried out by various catalyst systems. MoCl5-based catalyst systems are more effective for the polymerization of 4,4-diphenoxy-1,6-heptadiyne than WCl6-based catalyst systems. Polymerization of 4,4-diphenoxy-1,6-heptadiyne leads to soluble, purple colored polymer with number average molecular weight (Mn) of 3.6x104-5.2x104.1H- and13C-MNR, IR,and UV-visible spectra of the resulting polymer indicated that poly(4,4-diphenoxy-1,6-hepatadiyne) possesses a polyene structure presumably with cyclic recurring units in the polymer backbone. Poly(4,4-diphenoxy-1,6-heptadiyne) had good thermal and oxidative stability, and good solubility in organic solvents. The electrical conductivity of iodine-doped polymer was 10−3-10−2 S/cm.
Similar content being viewed by others

References
For reviews, see: T. Masuda and T. Higashimura, Adv. Polym. Sci., 81, 121 (1986).
C. I. Simionescu and B. Percec, Prog. Polym. Sci. 8, 133 (1982).
T. Masuda, B. Z. Tang, T. Higashimura and H. Yamaoka, Macromolecules., 18,2369 (1985).
Y. H. Kim, Y. S. Gal, U. Y. Kim and S. K. Choi, Macromolecules., 21, 1991 (1988).
M. S. Chang, S. K. Kwon, and S. K. Choi, Macromolecules., 23, 4135 (1990).
M. S. Ryoo, W. C. Lee and S. K. Choi, Macromolecules. 23, 3029 (1990).
S. H. Han, U. Y. Kim, Y. S. Kang and S. K. Choi, Macromolecules., 24, 973 (1991).
Polymerization. A solution of MoCl5 (0.073 ml, 0.2 M chlorobenzene solution) is added to a solution of DPHD (0.2 g) and chlorobenzene (7.3 ml, [M]0=0.1) at room temperature. The polymerization was carried out at 60 °C for 24 h, and the polymerization was terminated by adding a small amount of methanol. The resulting polymer was precipitated with a large excess of diethyl ether. Polymer yield was determined by gravimetry.
Y. H. Kim, K. Y. Choi and S. K. Choi, J. Polym. Sci. Polym. Lett. Ed., 27, 443 (1989).
O. K. Cho, Y. H. Kim, K. Y. Choi and S. K. Choi, Macromolecules., 23, 12 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ahn, HK., Kim, YH., Jin, SH. et al. Cyclopolymerization of 4,4-diphenoxy-1,6-heptadiyne by transition metal catalysts. Polymer Bulletin 29, 625–632 (1992). https://doi.org/10.1007/BF01041147
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01041147