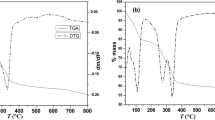
Abstract
Three isomers of naphthalenedisulfonates (naphthalene-2,6-,-1,5- and -2,7-disulfonates; abbreviated as N26DS, N15DS and N27DS, respectively, and collectively abbreviated as NijDS) are intercalated in the interlayer region of Zn and Al layered double-hydroxides (Zn/Al-LDH). The intercalated products are obtained under weak alkaline conditions as precipitates from reaction mixtures containing one of the isomers and calcined powder prepared by using carbonate-intercalated Zn/Al-LDH. When N26DS or N15DS is intercalated, two solid phases are observed which have different values of basal spacing in powder X-ray diffraction (XRD) patterns, whereas only one phase appears when N27DS is intercalated. The different basal spacing results from one of two alternate orientations taken by the interlayer NijDS. One of the two phases appears exclusively in XRD patterns, although the other is produced together with a by-product. A more closely packed orientation is suggested for the interlayer guest molecules in the latter phase. The solid state chemistry has been investigated using UV visible diffuse reflection (DR) spectroscopy, differential thermal analysis/thermogravimetry (DTA/TG) and X-ray photoelectron spectroscopy (XPS).
Similar content being viewed by others
References
F. Cavani, F. Trifiro, and A. Vaccari:Catalysis Today 11, 173 (1991); A. Roy, C. Forano, K.E. Malki, and J. Besse: in M.L. Occelli and H. Robson (ed.),Expanded Clays and Other Microporous Solids, Ch. 7, Van Nostrand Reinhold, New York (1992).
S. Miyata:Clays Clay Miner. 23, 369 (1975).
A. Bhattacharyya and D.B. Hall:Inorg. Chem. 31, 3869 (1992).
M.A. Drezdzon:Inorg. Chem. 27, 4628 (1988).
E. Kanezaki, K. Kinugawa, and Y Ishikawa:Chem. Phys. Lett. 226, 325 (1994).
K. Chibwe and W. Jones:J. Chem. Soc., Chem. Commun. 926 (1989).
I.Y. Park, K. Kuroda, and C. Kato:Chem. Lett. 2057 (1989).
K.R. Franklin, E. Lee, and C.C. Nunn:J. Mater Chem. 5, 565 (1995).
M. Meyn, K. Beneke, and G. Lagaly:Inorg. Chem. 29, 5201 (1990).
E. Kanezaki, M. Sugiyama, and Y. Ishikawa:J. Mater. Chem. 5, 1969 (1995).
E. Kanezaki, T. Sakamoto, A. Ookubo, and K. Ooi:J. Chem. Soc., Faraday Trans. 88, 3583 (1992).
MOPAC ver. 5.00 (QCPE No. 455), J.J.P. Steward:QCPE Bull. 9, 10 (1989); T. Hirano:JCPE Newsletter 1, 36 (1989); revised as Ver. 5.01 by J. Toyota for Apple Macintosh.
R. Allman:Chimica 24, 99 (1970);Acta Crystallogr B24, 972 (1968).
A. Streitwieser, Jr.:Molecular Orbital Theory for Organic Chemists, Ch. 8, Wiley, New York (1961).
D. Briggs and M.P. Seah:Practical Surface Analysis Auger and X-Ray Photoelectron Spectroscopy, Wiley, New York (1982).
W.T. Reichie, S.Y. Kang, and D.S. Everhardt:J. Catal. 101, 352 (1986).
D.D. Elleman and D. Williams:J. Chem. Phys. 25, 742 (1956).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kanezaki, E. Variable interlayer distance of intercalates of naphthalenedisulfonate isomers in hydrotalcite-like Zn and Al layered double-hydroxides. J Incl Phenom Macrocycl Chem 24, 341–351 (1996). https://doi.org/10.1007/BF01041118
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01041118