Summary
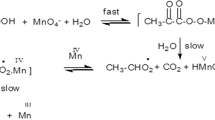
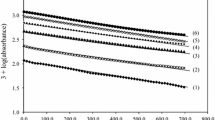
The kinetics of oxidation of leucine (LCO2H) by acid permanganate have been followed spectrophotometrically at 525 nm for the disappearance of MnVII and at 420 nm for the appearance of MnIV. The latter absorbance passes through a maximum, signifying the presence of a consecutive reaction involving a MnIV intermediate. The reaction is first order with respect to [MnO −4 ]. The rate constant, k7, has been evaluated at different [LCO2H], [H+] and temperatures, from plots of A 525corr versus time. The overall rate expression satisfying the observed kinetic parameters is
It is found that 8 moles of CO2 are produced and 8 moles of leucine are consumed per mole of permanganate consumed. The decarboxylation involves a cyclic chain reaction.
Similar content being viewed by others
References
P. P. Cohen,J. Biol. Chem.,119, 333 (1937).
D. E. Green, S. Mii and H. R. Mahler,J. Biol. Chem.,206, 1 (1954).
O. Toussaint, P. Capdevielle and M. Mansuy,Tetrahedron Lett. 25, 3819 (1984).
A. E. Braunstein and M. G. Kritzman,Nature (London),140, 503 (1937).
D. Shemin and R. M. Herbst,J. Am. Chem. Soc.,60, 1951 (1938);J. Biol. Chem.,147, 541 (1943).
B. T. Gowda and D. S. Mahadevappa,J. Chem. Soc., Perkin II, 323 (1983).
M. S. Ramachandran, T. S. Vivekanandam and R. P. M. M. Raj,J. Chem. Soc., Perkin II, 1345 (1984).
M. S. Ramachandran, T. S. Vivekanandam,J. Chem. Soc., Perkin II, 1341 (1984).
B. T. Gowda and R. Vijaya Laxmi Rao,Indian J. Chem.,24A, 1021 (1985).
M. B. Lal and K. K. Banerji,Tetrahedron,41, 6047 (1985).
K. Singh, A. K. Bose, J. N. Tiwari and S. P. Mushran,Ind. J. Chem.,16A, 35 (1978).
M. Bhargava, B. Sethuram and T. Navneeth Rao,Ind. J. Chem.,16A, 651 (1978).
R. A. Sheikh and W. A. Waters,J. Chem. Soc. (B), 968 (1970).
R. S. Verma, M. J. Reddy and V. R. Shastry,J. Chem. Soc. Perkin Trans. II, 469 (1976).
O. G. Pokrovskaya,Izvest Sibirsk, Otdel Akad, Nauk, S.S.S.R.,8, 50 (1959).
J. W. Ladbury and C. F. Cullis,Chem. Rev.,58, 403 (1958).
L. I. Simandi, M. Jaky, C. R. Savage and Z. A. Schelly,J. Am. Chem. Soc.,107, 4220 (1985).
F. Freeman, L. Y. Chang, C. J. Kappas and L. Sumarta,J. Org. Chem.,52, 1460 (1987).
N. Ganapathisubramanian,J. Phys. Chem.,92, 414 (1988).
G. Chandra and S. N. Srivastava,Ind. J. Chem.,11, 773 (1973).
V. S. Rao, B. Sethuram and T. Navneeth Rao,Int. J. Chem. Kinetic,11, 165 (1979).
A. I. Vogel,A Textbook of Quantitative Inorganic Analysis, Longmans Green, London, p. 282, 1964.
F. Fiegl,Spot Test in Organic Analysis, Elsevier, Amsterdam, p. 195, 1966.
M. Yousuf Hussain and Firoz Ahmad,J. Oxidn. Comm.,12, 59 (1989).
M. Yousuf Hussain and Firoz Ahmad,Transition. Metal. Chem.,14, 169 (1989).
R. M. Noyes and D. J. Sibbett,J. Am. Chem. Soc.,75, 767 (1953).
A. W. Adamson,J. Phys. and Colloid Chem., 5293 (1951).
F. C. Tompkins,Trans. Faraday Soc.,38, 128 (1942).
D. Neil Furlong, D. Wells and H. F. Sosse Wolfgang,Aust. J. Chem.,39, 769 (1986).
R. Stewart,Oxidation Mechanisms Applications to Organic Chemistry, W. A. Benjamin, New York, 1964, pp. 103, 177 and 128.
D. S. Mahadevappa, K. S. Rangappa, N. M. M. Gowda and B. T. Gowda,J. Phys. Chem.,85, 3651 (1981).
J. R. Sutter and K. B. Park,J. Phys. Chem.,88, 770 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hussain, M.Y., Ahmad, F. Kinetics and mechanism of oxidation of DL-leucine by acid permanganate. Transition Met Chem 15, 185–190 (1990). https://doi.org/10.1007/BF01038373
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01038373