Summary
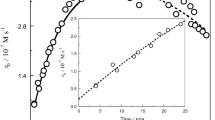
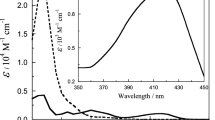
Kinetics of the oxidation of Co(cydta)2− (H4cydta=trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid) by peroxodisulphate (S2O 2−8 ) and by hydrogen peroxide have been studied in the ranges of pH 3.6–5.8 (acetate buffer) and 6.0–8.0 (phosphate buffer), respectively. For the first oxidant, the reaction was shown to exhibit second-order kinetics, first-order in each of the reactants. No pH dependence on rate was observed. The hydrogen peroxide reaction was also second-order, being first-order in each of the reactants. The rate of reaction showed inverse [H+] dependence. The rate law is given by\({\text{d[Co(cydta)}}^-- {\text{]/dt = 2\{ k}}_{{\text{H}}_{\text{2}} O_2 } {\text{ + k}}_{{\text{H}}O_2 - } {\text{K}}_{\text{a}} {\text{[H}}^{\text{ + }} {\text{]}}^{--1} {\text{\} [Co(cydta)}}^{{\text{2}}--} {\text{][H}}_{\text{2}} {\text{O}}_{\text{2}} {\text{]}}\). At 30 °C (I, 0.5 M), the ratio of\(k_{HO_2 - } /k_{H_2 O_2 } \sim 10^5 \). The corresponding activation parameters obtained from the temperature-dependence of rate are ΔH≠= (136±9) kJ mol−1, ΔS≠=(119±10) JK−1 mol−1 (for the peroxodisulphate system) and ΔH≠=(75±2) kJ mol−1, ΔS≠ =(−78±4) JK−1 mol−1 (for the hydrogen peroxide system).
Similar content being viewed by others
References
P. Banerjee and M. P. Pujari,Z. Anorg. Allgm. Chem., 473, 224 (1981).
P. Banerjee and M. P. Pujari,Transition Met. Chem., 6, 47 (1981).
P. Banerjee and M. P. Pujari,Bull. Chem. Soc. Jpn., 54, 2496 (1981).
P. Banerjee and M. P. Pujari,Indian J. Chem., (in press).
F. P. Dwyer and F. L. Garvan,J. Am. Chem. Soc., 83, 2610 (1961).
B. B. Smith and R. M. Betts,Inorg. Chem., 9, 2585 (1970).
D. H. Huchital and R. J. Hodges,Inorg. Chem., 12, 998 (1973).
S. S. Gupta and Y. K. Gupta,Inorg. Chem., 20, 454 (1981).
K. Ohashi, M. Matsuzawa, E. Hamano and K. Yamamoto,Bull. Chem. Soc. Jpn., 49, 2440 (1976).
J. O. Edwards inPeroxide Reaction Mechanisms, Interscience, New York (1961).
J. L. Sudmeier and G. Occupati,Inorg. Chem., 7, 2524 (1968).
J. L. Sudmeier, A. J. Senzel and G. L. Blackmer,Inorg. Chem., 10, 90 (1971).
P. Banerjee and M. P. Pujari, Unpublished results.
S. Funahashi, K. Haraguchi and M. Tanaka,Inorg. Chem., 16, 1349 (1977).
S. Funahashi, K. Ishihara and M. Tanaka,Inorg. Chem., 20, 51 (1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pujari, M.P., Banerjee, P. Kinetics of the peroxodisulphate and hydrogen peroxide oxidations oftrans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetatocobaltate(II) in acidic and basic buffer media. Transition Met Chem 8, 91–93 (1983). https://doi.org/10.1007/BF01036087
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01036087