Summary
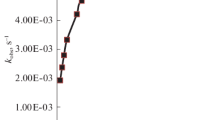
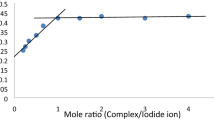
The bromate ion reduction by 12-tungstocobaltate(II) anion has been investigated. The reaction obeys the empirical rate law:-d[reductant]/dt=5(a+b[H+]2)[BrO −3 ][reductant]: where a=(2.49±0.18)×10−4M−1 s−1, b=(4.65±0.20)×10−5M−3s−1 at 24.5±0.1°C [H+]=0.05–1.50M and I=2.0M (NaClO4). This rate law is interpreted in terms of parallel reactions of BrO −3 and H2BrO +3 . On the basis of the observed anion catalysis, substitution intertness of the reductant and Marcus type linear free energy relations, the outer sphere mechanism is proposed for both pathways.
Similar content being viewed by others
References
G. A. Ayoko and M. A. Olatunji,Polyhedron,2, 577 (1983).
G. A. Ayoko and M. A. Olatunji,Inorg. Chim. Acta,80 L15 (1983);ibid,Inorg. Chim. Acta,80, 287 (1983).
G. A. Ayoko and M. A. Olatunji,Polyhedron,3, 191 (1984).
G. A. Ayoko and M. A. Olatunji,Gazz. Chim. Ital.,21, 114 (1984).
M. A. Olatunji and G. A. Ayoko,Bull. Soc. Chim., FR.,5, 705 (1985).
G. A. Ayoko and M. A. Olatunji,Transition Met. Chem.,10, 7 (1985).
C. H. Brubaker and P. G. Rasmussen,Inorg. Chem.,3, 977 (1964).
M. T. Tope and G. M. Varga Jr.,Inorg. Chem.,5, 1249 (1966).
L. C. W. Baker and T. P. McCutcheon,J. Am. Chem. Soc.,78 4503 (1956).
L. C. W. Baker and V. E. Simmons,J. Am. Chem. Soc.,81, 4744 (1959).
S. K. Saha, M. C. Ghosh and P. Banerjee,Inorg. Chem. Acta,126, 26 (1987);J. Chem. Soc. Dalton Trans., 1301 (1986).
J. P. Birk,Inorg. Chem.,12, 2468 (1973).
R. C. Thompson,Inorg. Chem.,10, 1892 (1971).
G. C. Knight and R. C. Thompson,Inorg. Chem.,12, 63, (1973).
J. P. Birk and S. G. Kozub,Inorg. Chem.,17 1186, (1978).
J. P. Birk,Inorg. Chem.,17, 504 (1978).
J. P. Birk and S. G. Kozub,Inorg. Chem.,12, 2460 (1973).
C. Sharp and A. G. Sykes,Inorg. Chem.,27, 501 (1988).
J. N. Bronsted,Z. Phys. Chem.,102, 160 (1922).
R. G. Wilkins,The Study of Kinetics and Mechanism of Reactions of Transition Metal Complexes, Allyn and Bacon Boston, P. 111 (1974).
R. G. Marcus,J. Phys. Chem.,67, 853 (1963);Electrochi. Acta,13, 995 (1968).
A. Adegite, J. F. Iyun and J. F. Ojo,J. Chem. Soc. Dalton Trans., 115 (1977) and ref. therein.
T. J. Przystas and N. Sutin,J. Am. Chem. Soc.,95, 5545 (1973).
D. E. Pennington and A. Haim,Inorg. Chem.,6, 2137 (1967).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ayoko, G.A., Iyun, J.F. & Faskari El-Idris, I. Electron transfer at tetrahedral cobalt(II). Part 1. Kinetics of bromate ion reduction. Transition Met Chem 16, 145–148 (1991). https://doi.org/10.1007/BF01032820
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01032820