Abstract
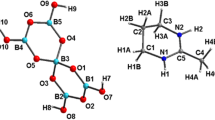
Synthetic methods are reported for the preparation of compounds containing the trinuclear triangular cluster [W3S4Br3(depe)3[+. These involve reactions between WBr5 and NaB(C2 H5)3H or NaBH4 as reducing agent in THF, and subsequent addition of methanolic solutions of NaHS and depe ligand. Both compounds, [W3S3Br3(depe)3]PF6·0.5C7H8,1, and [W3S4Br3(depe)3]Br·2CH3OH,2, are characterized by x-ray single crystal studies. Compounds1 and2 crystallize in space group\(P\bar 1\). For1,a=10.427 (3) Å,b=15.415(4) Å,c=18.140(5) Å, α=79.36(2)°, β=73.59(2)°, γ=81.54(2)°, andV=2734.8(2) Å3;R=0.050 and for 2a=10.491(3) Å,b=15.074(3) Å,c=18.246 Å, α=95.76(2)°, β=105.82(2)°, γ=98.18(2)°, andV=2718.4(3) Å3;R=0.081. The two cations in1 and2 possess C3 symmetry. The W-W distances are in the range 2.783−2.891 Å (for1) and 2.778−2.785 Å (for2) and the average W-Br distances in1 and2 are 2.616[2] Å and 2.594[4] Å, respectively. Each metal atom in the [W3S4Br3(depe)3]+ ions is attached to one capping sulfur atom, two bridging sulfur atoms, one bromine atom, and one chelating depe ligand. One P atom in depe ligand istrans to μ3-S and the otherP atom istrans to a μ2-S atom. UV-Vis and NMR spectra for these compounds are also reported.
Similar content being viewed by others
References
H. M. Spittle and W. Wardlaw (1929).J. Chem. Soc. 792.
W. H. McCarroll, L. Katz, and R. Ward (1957).J. Am. Chem. Soc. 79, 5410;
G. B. Ansell and Katz (1966).Acta Crystallogr. 21, 482;
M. Herceg and S. Scavnicar (1967).Croat. Chem. Acta 39, 137.
F. A. Cotton (1964).Inorg. Chem. 3, 1217.
A. Bino, F. A. Cotton, and Z. Dori (1978).J. Am. Chem. Soc. 100, 5252.
T. Shibahara, H. Akashi, S. Nagahata, H. Hattori, and H. Kuroya (1989).Inorg. Chem. 28, 362;
D. T. Richens, L. Helm, P.-A. Pittet, A. E. Merbach, F. Nicolo, and G. Chapius (1989).Inorg. Chem. 28, 1394;
B.-L. Ooi, C. Sharp, and A. G. Sykes (1989).J. Am. Chem. Soc. 111, 125.
T. Shibahara, K. Kohda, A. Ohtsuji, K. Yasuda, and H. Kuroya (1986).J. Am. Chem. Soc. 108, 2757.
T. Shibahara, A. Takcuchi, A. Ohtsuji, K. Kohda, and H. Kuroya (1987).Inorg. Chim. Acta 127, L45.
V. P. Fedin, M. N. Sokolov, O. A. Geras'ko, M. Sheer, V. Ye. Federov, A. V. Mironov, Yu. L. Slovohotov, and Yu. T. Struchkov (1989).Inorg. Chim. Acta 165, 25.
F. A. Cotton and R. Llusar (1988).Inorg. Chem. 27, 1303;
F. A. Cotton, R. Llusar, and C. T. Eagle (1989).J. Am. Chem. Soc. 111, 4332.
F. A. Cotton, P. A. Kibala, and C. S. Miertschin (1991).Inorg. Chem. 30, 548.
F. A. Cotton, P. A. Kibala, M. Matusz, C. S. McCaleb, and R. B. W. Sandor (1989).Inorg. Chem. 28, 2623.
R. E. Eibeck (1963).Inorg. Syn. 7, 128.
A. Bino, F. A. Cotton, and P. E. Fanwick (1979).Inorg. Chem. 18, 3558;
F. A. Cotton, B. A. Frenz, G. Deganello, and A. J. Shaver (1973).J. Organomental. Chem. 50, 277.
A. C. T. North, D. C. Phillips, and F. S. Mathews (1968).Acta Crystallogr. Sect. A: Cryst. Phys. Diffr. Theor. Gen. Crystallogr. Sect. A: Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 24, 351.
A. Müller, R. Jostes, and F. A. Cotton (1980).Angew. Chem. Int. Ed. Engl. 19, 875.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cotton, F.A., Mandal, S.K. & Shang, M. New synthetic routes for the preparation of the triangular trinuclear clusters of tungsten-containing bromine atoms as terminal ligands: Structural characterization of [W3S4Br3(depe)3]PF6·0.5C7 H8 and [W3S4Br3(depe)3]Br·2CH3OH. J Clust Sci 1, 287–305 (1990). https://doi.org/10.1007/BF01032274
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01032274