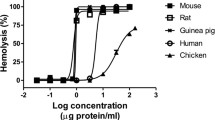
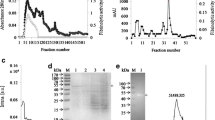
Abstract
A hemocyte lysate from horseshoe crab produced a gel, when exposed to Gram-negative bacterial endotoxins. This gelation reaction of the lysate, so-called Limulus test, has been widely employed as a simple and very sensitive assay method for endotoxins. Recent biochemical studies on the principle of Limulus test indicate that the hemocytes contain several serine protease zymogens, which constitute a coagulation cascade triggered by endotoxins, and that there is a (1 → 3)-β-d-glucan-mediated coagulation pathway which also results in the formation of gel. Up to now, six protein components, designated coagulogen, proclotting enzyme, factor B, factor C, factor G and anti-LPS factor, all of which are closely associated with the endotoxin-mediated coagulation pathway, have been purified and biochemically characterized. Among these components, the complete amino acid sequences of coagulogens isolated from one American and three Asian species of horseshoe crabs have been established. Moreover, the reconstitution experiment using the isolated clotting factors, C, B, proclotting enzyme and coagulogen in the presence of endotoxin, leads to the formation of coagulin get. Based on these results, we propose here a mechanism for the Limulus coagulation cascade.
Similar content being viewed by others
References
Aketagawa, J., Miyata, T., Ohtsubo, S., Nakamura, T., Morita, T., Hayashi, H., Miyata, T., Iwanaga, S., Takao, T., and Shimonishi, Y. (1986).J. Biol. Chem. 261, 7357–7365.
Dumont, J. N., Anderson, E., and Winner, G. (1966).J. morphol. 119, 181–208.
Fries, C. R. (1984). InComparative Pathology, Vol. 6 (Cheng, T. C., ed.), Plenum Press, New York, pp. 49–109.
Harada-Suzuki, T., Morita, T., Iwanaga, S., Nakamura, S., and Niwa, M. (1982).J. Biochem. 92, 793–800.
Levin, J., and Bang, F. B. (1964).Bull. Johns Hopkins Hosp. 115, 265–274.
Miyata, T., Usui, K., and Iwanaga, S. (1984a).J. Biochem. 95, 1793–1801.
Miyata, T., Hiranaga, M., Umezu, M., and Iwanaga, S. (1984b).J. Biol. Chem. 259, 8924–8933.
Miyata, T., Matsumoto, H., Hattori, M., Sakaki, Y., and Iwanaga, S. (1986).J. Biochem.,100, 213–220.
Morita, T., Tanaka, S., Nakamura, T., and Iwanaga, S. (1981).FEBS Lett. 129, 318–321.
Morita, T., Ohtubo, S., Nakamura, T., Tanaka, S., Iwanaga, S., Ohashi, K., and Niwa, M. (1985a).J. Biochem. 97, 1611–1620.
Morita, T., Nakamura, T., Miyata, T., and Iwanaga, S. (1985b).Prog. Clin. Biol. Res. 189, 53–64.
Mosesson, M. W., Wolfenstein-Todel, C., Levin, J., and Bartrand, O. (1979).Thromb. Res. 14, 765–779.
Müller, E. H., Levin, J., and Holme, R. (1975).J. Cell Physiol. 816, 533–543.
Nakamura, S., Takagi, T., Iwanaga, S., Niwa, M., and Takahashi, K. (1976a).J. Biochem. 80, 649–652.
Nakamura, S., Iwanaga, S., Harada, T., and Niwa, M. (1976b).J. Biochem. 80, 1011–1021.
Nakamura, S., Takagi, T., Iwanaga, S., Niwa, M., and Takahashi, K. (1976c).Biochem. Biophys. Res. Commun. 72, 902–908.
Nakamura, S., Morita, T., Iwanaga, S., Niwa, M., and Takahashi, T. (1977).J. Biochem. 81, 1567–1569.
Nakamura, S., Morita, T., Harada-Suzuki, T., Iwanaga, S., Takahashi, K., and Niwa, M. (1982).J. Biochem. 92, 781–792.
Nakamura, T., Morita, T., and Iwanaga, S. (1985).J. Biochem. 97, 1561–1574.
Nakamura, T., Morita, T., and Iwanaga, S. (1986a).Eur. J. Biochem.,154, 511–521.
Nakamura, T., Horiuchi, T., Miyata, T., and Iwanaga, S. (1986b).J. Biochem.,99, 847–857.
Niwa, M., and Waguri, O. (1974).Saikagaku 47, 1–13.
Ohashi, K., Niwa, M., Nakamura, T., Morita, T., and Iwanaga, S. (1984).FEBS Lett. 176, 207–210.
Ohki, M., Nakamura, T., Morita, T., and Iwanaga, S. (1980).FEBS Lett. 120, 217–220.
Ornberg, R. L., and Reese, T. S. (1979).Prog. Clin. Biol. Res. 29, 125–130.
Solum, N. O. (1973).Thromb. Res. 2, 55–70.
Spurling, N. W. (1981).Comp. Biochem. Physiol. 68A, 541–548.
Srimal, S., Miyata, T., Kawabata, S., Miyata, T., and Iwanaga, S. (1985).J. Biochem. 98, 305–318.
Tanaka, S., Nakamura, T., Morita, T., and Iwanaga, S. (1982).Biochem. Biophys. Res. Commun. 105, 717–723.
Takagi, T., Hokama, Y., Miyata, T., Morita, T., and Iwanaga, S. (1984).J. Biochem. 95, 1445–1457.
Tay, J. Y., Seid, R. C., Huhn, R. D., and Liu, T.-Y. (1977).J. Biol. Chem. 255, 4773–4776.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Iwanaga, S., Morita, T., Miyata, T. et al. The hemolymph coagulation system in invertebrate animals. J Protein Chem 5, 255–268 (1986). https://doi.org/10.1007/BF01025424
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01025424