Summary
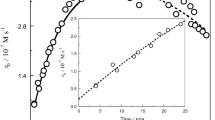
The kinetics and mechanism of exchange of HPDTA in [Fe2HPDTA(OH)2] with cyanide ion (HPDTA=2-hydroxytrimethylenediaminetetraacetic acid) was investigated spectrophotometrically by monitoring the peak at 395 nm (λ max of [Fe(CN)5OH]3− at pH=11.0±0.02,I=0.25m (NaClO4) at ±0.1°C).
Three distinct observable stages were identified; the first is the formation of [Fe(CN)5OH]3−, the second the formation of [Fe(CN)6]3− from it and the third the reduction of [Fe(CN)6]3− to [Fe(CN)6]4− by HPDTA4− released in the first stage.
The first stage follows first-order kinetics in [Fe2HPDTA(OH)2] and second-order in [CN−] over a wide range of [CN−], but becomes zero order at [CN−]<5×10−2 m. We suggest a cyanide-independent dissociation of [Fe2HPDTA)(OH)2] into [FeHPDTA(OH)] and [Fe(OH)]2+ at low cyanide concentrations and a cyanide-assisted rapid dissociation of [Fe2HPDTA(OH)2] to [FeHPDTA(OH)(CN)]3− and [Fe(OH)]2+ at higher cyanide concentrations. The excess of cyanide reacts further with [FeHPDTA(OH)(CN)]3− finally to form [Fe(CN)5OH]3−.
The reverse reaction between [Fe(CN)5OH]3− and HPDTA4− is first-order in [Fe(CN)5OH]3− and HPDTA4−, and exhibits inverse first-order dependence on cyanide concentration.
A six-step mechanism is proposed for the first stage of reaction, with the fifth step as rate determining.
Similar content being viewed by others
References
H. C. Bajaj and P. C. Nigam,Transition Met. Chem.,8, 105 (1983).
R. M. Naik and P. C. Nigam,Transition Met. Chem.,10, 227 (1985).
R. M. Naik and P. C. Nigam,Inorg. Chim. acta,114, 55 (1986).
R. M. Naik and P. C. Nigam,Inorg. Chim. Acta,127, 7 (1987).
R. M. Naik and P. C. Nigam,Transition Met. Chem.,11, 337 (1986).
L. G. Sillen and A. E. Martell,Stability Constants of Metalion Complexes, Suppl. No. 1, The Chem. Soc., London, 1971, p. 768.
K. S. Klausen and O. E. Rudd,Anal. Chim. Acta.,57, 351 (1971).
D. G. Lambert and M. M. Jones,J. Am. chem. Soc.,88, 4615 (1966).
Nishi Gupta, R. M. Naik and P. C. Nigam,Ind. J. Chem.,25A, 39 (1986).
A. I. Vogel,A Textbook of Micro- and Macro-qualitative Analsis, Longman Green and Co, London, 4th Edit., 1954, pp. 348, 349.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Naik, R.M., Nigam, P.C. Kinetic and mechanism of pentacyanohydroxoferrate(III) formation from the reaction of [Fe2HPDTA(OH)2] with cyanide ions. Transition Met Chem 12, 261–264 (1987). https://doi.org/10.1007/BF01023544
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01023544