Abstract
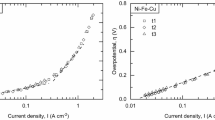
The exceptional activity of carbon anodes for the fluorine evolution reaction (FER) in a KF·2HF melt at 358 K that can be produced, based on a previously reported procedure for reactivation following onset of the ‘anode effect’, has been characterized. The activated carbon anodes that have been treated by applying the procedure exhibit unique, exceptionally active, polarization behaviour. This is manifested as a high exchange current-density, a low Tafel slope in the high current-density range, resistance to further anodic effects, facilitation of the detachment of F2 gas bubbles and good electrode-life properties. At polarization current-densities of 0.1 and 0.2 A cm−2, the anode potential is 1.5 and 2.5 V lower, respectively, for the activated that for a normal non-activated carbon electrode. The improvements of the activated carbon anodes are associated with facilitation of the detachment of the F2 bubble/film. The F2 bubble/film adherence effect is the main cause for the abnormally high polarization and anode effect for a normal, unactivated carbon anode, as concluded in our previous studies (Part I). It is shown that the facilitation of F2 bubble/film detachment is due to two physical properties of the activated carbon electrode: a much smaller solid/gas/liquid contact angle and a much smoother surface. As determined by means of ESCA, it is shown that, compared with an unactivated carbon anode, the activated anodes have a smaller extent of surface fluorination corresponding to thinner ‘CF’ films. This may lead both to a favourable contact angle and a smaller barrier layer for activated electron tunnelling at the activated carbon anodes used for the FER, than at normal non-activated carbon electrodes.
Similar content being viewed by others
References
Lijun Bai and B. E. Conway,J. Applied Electrochem. 18 (1988) 839.
,20 (1990) 916.
W. Rüdorff, U. Hoffman, G. Rüdorff, J. Endell and G. Ruess,Z. Anorg. Allgem. Chem. 256 (1948) 125.
W. V. Childs and F. N. Ruehlen, US Patent No. 3616336 (1971).
W. V. Childs, US Patent No. 3616315 (1971).
P. T. Hough and D. M. Novak-Antoniou, US Patent No. 4602 985 awarded to Eldorado Resources Ltd. (1986).
D. M. Novak and P. T. Hough,J. Electroanal. Chem. 144 (1983) 121.
N. Watanabe, M. Ishii and S. Yoshizawa,J. Electrochem. Soc. Jap. 29 (1961) E180; see also N. Watanabe,Proc. Electrochem. Soc. ‘Electrochemistry of Carbon’ (1984) 536.
O. R. Brown, B. M. Ikeda and M. J. Wilmott,Electrochim. Acta 32 (1987) 1163.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bai, L., Conway, B.E. Electrochemistry of anodic fluorine gas evolution at carbon electrodes: Part III characterization of activated carbon anodes following onset of the ‘anode effect’. J Appl Electrochem 20, 925–931 (1990). https://doi.org/10.1007/BF01019567
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01019567