Abstract
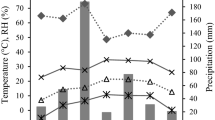
Astringency (tannin content) of Douglas-fir [Pseudotsuga menziesii (Mirb.) Franco] foliage was examined in relation to phenology and water status. Foliage was sampled from control trees, trees on south-facing slopes, and trenched trees prior to budbreak and at periods of two, six, and 12 weeks after budbreak. Astringency of prebudbreak foliage from untrenched trees was comparable to that of mature foliage. Foliage expansion was accompanied by dilution of the tannin content. Percent relative astringency of control trees was significantly and positively related to the absolute value of predawn xylem pressure potential, while this relationship was negative for trees in the south-facing group. Coefficients of determination for the relationship between astringency and predawn xylem pressure potential were high (0.67 and 0.79 for the control and south-facing groups, respectively). Astringency of foliage from trees in the south-facing group also was affected significantly by daytime xylem pressure potential. Astringency of foliage from trees in the control and south-facing groups was not significantly related to tissue age, while that from trenched trees was significantly related only to age. Results demonstrate that water status is a better predictor of foliage astringency than is tissue age in unperturbed trees of this species and that, depending on the magnitude and/or timing of water deficits, opposite relationships between astringency and xylem pressure potential can be observed.
Similar content being viewed by others
References
Asquith, T.N., andButler, L.G. 1986. Interactions of condensed tannins with selected proteins.Phytochemistry 25:1591–1593.
Beart, J.E., Lilley, T.H., andHaslam, E. 1985. Plant polyphenols—secondary metabolism and chemical defence: some observations.Phytochemistry 24:33–38.
Crankshaw, D.R., andLangenheim, J.H. 1981. Variation in terpenes and phenolics through leaf development inHymenaea and its possible significance to herbivory.Biochem. Syst. Ecol. 9:115–124.
Dement, W.A., andMooney, H.A. 1974. Seasonal variation in the production of tannins and cyanogenic glucosides in the chaparral shrub,Heteromeles arbutifolia.Oecologia 15:65–76.
Feeny, P. 1970. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars.Ecology 51:565–581.
Feeny, P. 1976. Plant apparency and chemical defense.Recent Adv. Phytochem. 10:1–40.
Feeny, P.P., andBostock, H. 1968. Seasonal changes in the tannin content of oak leaves.Phytochemistry 7:871–880.
Floate, M.J.S. 1970. Decomposition of organic material from hill soils and pastures. IV. The effects of moisture content on the mineralisation of carbon, nitrogen, and phosphorus from plant materials and faeces.Soil Biol. Biochem. 2:275–283.
Gershenzon, J. 1984. Changes in the levels of plant secondary metabolites under water and nutrient stress, pp. 273–320,in B.N. Timmermann, C. Steelink, and F.A. Loewus (eds.). Phytochemical Adaptations to Stress. Plenum Press, New York.
Goldstein, J.L., andSwain, T. 1963. Changes in tannins in ripening fruits.Phytochemistry 2:371–383.
Guinn, G., andEidenbock, M.P. 1982. Catechin and condensed tannin contents of leaves and bolls of cotton in relation to irrigation and boll load.Crop Sci. 22:614–616.
Haslam, E. 1981. Vegetable tannins, pp. 527–556,in E.E. Conn and P.K. Stumpf (eds.). The Biochemistry of Plants: A Comprehensive Treatise, Vol. 7. Secondary Plant Products. Academic Press, New York.
Martin, J.S., andMartin, M.M. 1982. Tannin assays in ecological studies: Lack of correlation between phenolics, proanthocyanidins and protein-precipitating constituents in mature foliage of six oak species.Oecologia 54:205–211.
Mooney, H.A.,Gulmon, S.L., andJohnson, N.D. 1983. Physiological constraints on plant chemical defenses, pp. 21–36,in P.A. Hedin (ed.). Plant Resistance to Insects. ACS Symposium Series, Washington, D.C.
Rhoades, D.F. 1979. Evolution of plant chemical defenses against herbivores, pp. 3–54,in G.A. Rosenthal and D.H. Janzen (eds.). Herbivores: Their Interaction with Secondary Plant Metabolites. Academic Press, New York.
Rhoades, D.F., andCates, R.G. 1976. Toward a general theory of plant antiherbivore chemistry.Recent Adv. Phytochem. 10:168–213.
Salisbury, F.B., andRoss, C.W. 1978. Plant Physiology, 2nd ed. Wadsworth Publishing, Belmont, California.
Schultz, J.C., Baldwin, I.T., andNothnagle, P.J. 1981. Hemoglobin as a binding substrate in the quantitative analysis of plant tannins.J. Agric. Food. Chem. 29:823–826.
Sokal, R.R., andRohlf, F.J. 1981. Biometry, 2nd ed. W.H. Freeman, New York.
Stafford, H.A., andCheng, T.-Y. 1980. The procyanidins of Douglas fir seedlings, callus and cell suspension cultures derived from cotyledons.Phytochemistry 19:131–135.
Stafford, H.A., andLester, H.H. 1980. Procyanidins (condensed tannins) in green cell suspension cultures of Douglas fir compared with those in strawberry and avocado leaves by means of C18-reversed-phase chromatography.Plant Physiol. 66:1085–1090.
Stafford, H.A., andLester, H.H. 1981. Proanthocyanidins and potential precursors in needles of Douglas fir and in cell suspension cultures derived from seedling shoot tissues.Plant Physiol. 68:1035–1040.
Swift, M.J., Heal, O.W., andAnderson, J.M. 1979. Decomposition in Terrestrial Ecosystems. University of California Press, Los Angeles.
Tempel, A.S. 1981. Field studies of the relationship between herbivore damage and tannin concentration in bracken (Pteridiumaquilinum Kuhn).Oecologia 51:97–106.
Wagner, M.R., 1986. Influence of moisture stress and induced resistance in ponderosa pine,Pinus ponderosa Dougl. ex. Laws, on the pine sawfly,Neodiprion autumnalis Smith.For. Ecol. Manage. 15:43–53.
Walters, T., andStafford, H.A. 1984. Variability in accumulation of proanthocyanidins (condensed tannins) in needles of Douglas-fir (Pseudotsuga menziesii) following long-term budworm defoliation.J. Chem. Ecol. 10:1469–1476.
Waring, R.H., andSchlesinger, W.H. 1985. Forest Ecosystems. Concepts and Management. Academic Press, New York.
White, T. 1957. Tannins—their occurrence and significance.J. Sci. Food Agric. 8:377–385.
Zar, J.H. 1974. Biostatistical Analysis. Prentice-Hall, Englewood Cliffs, New Jersey.
Zucker, W.V. 1983. Tannins: does structure determine function? An ecological perspective.Am. Nat. 121:335–365.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Horner, J.D. Astringency of douglas-fir foliage in relation to phenology and xylem pressure potential. J Chem Ecol 14, 1227–1237 (1988). https://doi.org/10.1007/BF01019348
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01019348