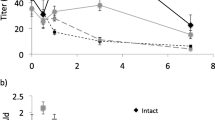
Abstract
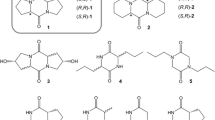
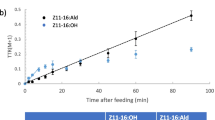
A series of mono-, di-, and trihalogenated acetate analogs of Zl 1–16: Ac were prepared and examined for electrophysiological activity in antennae of males of the diamondback moth,Plutella xylostella. In addition, two potential affinity labels, a diazoacetate (Dza) and a trifluoromethyl ketone (Tfp), were evaluated for EAG activity. The Z11–16∶Ac showed the highest activity in EAG assays, followed by the fluorinated acetates, but other halo-acetates were essentially inactive. The polar diazoacetate and the trifluoromethyl ketone were also very weak EAG stimulants. The effects of these analogs on the hydrolysis of [3H]Z11–16∶Ac to [3H]Z11–16∶OH by antennal esterases was also examined. The three fluorinated acetates showed the greatest activity as inhibitors in competition assays, with rank order F2Ac > F3Ac > FAc > Ac > Cl2Ac > ClAc > Dza > Br2Ac > BrAc > Tfp > I > Cl3Ac > Br3Ac > OH. The relative polarities of the haloacetates, as determined by TLC mobility, are in the order mono- > di- > trihalo, but F, Cl, Br, and I all confer similar polarities within a substitution group. Thus, the steric size appears to be the predominant parameter affecting the interactions of the haloacetate analogs with both receptor and catabolic proteins inP. xylostella males.
Similar content being viewed by others
References
Albans, K.R., Baker, R., Jones, O.T., Justum, A.R., andTurnbull, M.D. 1984. Inhibition of response ofHeliothis virescens to its natural pheromone by antipheromones.Crop Prot. 3:501–506.
Baker, R.,Green, A.,Justum, A.R., andTurnbull, M.D. 1981. Protecting plants from insect pests. Eur. Pat. Appl. EP 42,228.
Bestmann, H.J. 1986. Synthesis, structure activity relationships and mode of action of insect pheromones, pp. 45–64,in Atta-ur-Rahman (ed.). Natural Products Chemistry. Springer-Verlag, Heidelberg.
Bestmann, H.J., andVostrowsky, O. 1982. Structure-activity relationships in insect pheromones. A dynamical model of pheromone interactions with receptor sites, pp. 253–263,in W. Breipohl (ed.). Olfaction and Endocrine Regulation. IRL Press, London.
Chisholm, M.D., Steck, W.F., Arthur, A.P., andUnderhill, E.W. 1975. Evidence for cis-11-hexadecen-1-ol acetate as a major component of the sex pheromone of the bertha army worm,Mamestra configurera (Lepidoptera: Noctuidae).Can. Entomol. 107:361–366.
Chisholm, M.D., Steck, W.F., Underhill, E.W., andPalaniswamy, P. 1983. Field-trapping of diamondback mothPlutella xylostella using an improved four-component sex attractant blend.J. Chem. Ecol. 9:113–118.
Chow, Y.S., Lin, Y.M. andHsu, C.T. 1977. Sex pheromone of the diamondback moth (Lepidoptera: Plutellidae).Bull. Inst. Zool. Acad. Sin. 16:99–105.
Corey, E.J., andMyers, A.G. 1984. Efficient synthesis and intramolecular cyclopropanation of unsaturated esters of diazoacetic acid.Tetrahedron Lett. 25:3559–3562.
De Kramer, J.J., andHemberger, J. 1987. The neurobiology of pheromone reception, pp. 433–471, in G.D. Prestwich and G.J. Blomquist (eds.). Pheromone Biochemistry. Academic Press, New York.
Ding, Y.-S., andPrestwich, G.D. 1986. Metabolic transformation of tritium-labeled pheromone by tissues ofHeliothis virescens moths.J. Chem. Ecol. 12:411–429.
Duncan, D.B. 1955. Multiple range and multiple F tests.Biometrics 11:1–41.
Ferkovich, S.M. 1981. Enzymatic alteration of insect pheromones, pp. 165–185,in D.M. Norris (ed.). Perception of Behavioral Chemicals. Elsevier/North Holland, Amsterdam.
Ferkovich, S.M., Oliver, J., andDillard, C. 1982a. Comparison of pheromone hydrolysis by the antennae with other tissues after adult eclosion in the cabbage looper moth,Trichoplusia ni.Entomol. Exp. Appl. 31:327–328.
Ferkovich, S.M., Oliver, J.E., andDillard, C. 1982b. Pheromone hydrolysis by cuticularand interior esterases of the antennae, legs, and wings of the cabbage looper moth,Trichoplusia ni (Hubner).J. Chem. Ecol. 8:859–866.
Ganjian, L., Pettei, M.J., Nakanishi, K., andKaissling, K.-E. 1978. A photoaffinity-labelled insect sex pheromone for the mothAntheraea polyphemus.Nature 271:157–158.
Hammock, B.D., Wing, K.D., Mclaughlin, J., Lovell, V., andSparks, T.C. 1982. Trifluoromethyl ketones as possible transition state analog inhibitors of juvenile hormone esterase.Pest. Biochem. Physiol. 17:76–88.
Henrick, C.A. 1977. The synthesis of insect pheromones.Tetrahedron 33:1845–1889.
Kafka, W.A., andNeuwirth, J. 1975. A model of pheromone molecule-acceptor interaction.Z. Naturforsch. 30c:278–282.
Kaissling, K.E. 1986. Chemo-electrical transduction in insect olfactory receptors.Annu. Rev. Neurosci. 9:121–145.
Klein, U. 1987. Sensillum lymph proteins from antennal olfactory hairs of the mothAntheraea polyphemus (Saturnidae)Insect Biochem. 17:1193–1204.
Klein, U., andKeil, T. A. 1984. Dendritic membrane from insect olfactory hairs: Isolation method and electron microscopic observations.Cell. Mol. Neurobiol. 4:385–396.
Liljefors, T., Thelin, B., andVander Pers, J.N.C. 1984. Structure-activity relationships between stimulus molecule and response of a pheromone receptor cell in turnip moth,Agrotis segetum: Modifications of the acetate group.J. Chem. Ecol. 10:1661–1675.
Lonergan, G. 1986. Metabolism of pheromone components and analogs by cuticular enzymes ofChoristoneura fumiferana.J. Chem. Ecol. 12:483–496.
Morse, D., andMeighen, E. 1984. Detection of pheromone biosynthetic and degradative enzymesin vitro.J. Biol. Chem. 259:475–480.
Morse, D., andMeighen, E. 1986. Pheromone biosynthesis and the role of functional groups in pheromone specificity.J. Chem. Ecol. 12:335–351.
Morse, D., andMeighen, E.A. 1987. Pheromone biosynthesis: Enzymatic studies in Lepidoptera, pp. 121–158,in G. D. Prestwich and G. J. Blomquist (eds.). Pheromone Biochemistry. Academic Press, New York.
Neises, B., andSteglich, W. 1978. A simple procedure for preparation of carboxylic esters.Angew. Chem. Int. Ed. Engl. 17:522–523.
Prestwich, G.D. 1986. Fluorinated sterols, hormones, and pheromones: Enzyme-targeted disruptants in insects.Pestic. Sci. 37:430–440.
Prestwich, G.D. 1987a. Chemical studies of pheromone reception and catabolism, pp. 473–527,in G.D. Prestwich and G.J. Blomquist (eds.). Pheromone Biochemistry. Academic Press, New York.
Prestwich, G.D. 1987b. Chemistry of pheromone and hormone metabolism in insects.Science. 237:999–1006.
Prestwich, G.D., andBlomquist, G.J. (eds.). 1987. Pheromone Biochemistry. Academic Press, New York, 546 pp.
Prestwich, G.D., Golec, F.A., andAndersen, N.H. 1984. Synthesis of a highly tritiated photoaffinity labeled pheromone analog for the mothAntheraea polyphemus.J. Labelled Compd. Radiopharm. 21:593–601.
Prestwich, G.D., Carvalho, J.F., Ding, Y.-S., andHendricks, D.E. 1986a. Acyl fluorides as reactive mimics of aldehyde pheromones: Hyperactivation and aphrodisia inHeliothis virescens.Experientia 42:964–966.
Prestwich, G.D., Vogt, R.G., andRiddiford, L.M. 1986b. Binding and hydrolysis of radiolabeled pheromone and several analogs by male-specific antennal proteins of the mothAntheraea polyphemus.J. Chem. Ecol. 12:323–333.
Prestwich, G.D., Vogt, R.G., andDing, Y.-S. 1987. Chemical studies of pheromone catabolism and reception, pp. 57–66,in J. Law (ed.). Molecular Entomology. UCLA Symposia on Molecular and Cellular Biology, New Series. Alan R. Liss, New York.
Priesner, E. 1979. Specificity studies on pheromone receptors of noctuid and tortricid Lepidoptera, pp. 57–71,in F.J. Ritter (ed.). Chemical Ecology: Odour Communication in Animals. Elsevier/North-Holland, Amsterdam.
Roelofs, W.L. 1984. Electroantennogram asssays: Rapid and convenient screening procedures for pheromones, pp. 131–160,in H.E. Hummel and T.A. Miller (eds.). Techniques in Pheromone Research. Springer-Verlag, New York.
Shaw, D.A., andTuominen, T.C. 1985. An efficient synthesis of 3-hydroxy-3-trifluoromethyl phthalide.Synth. Commun. 15:1291–1297.
Steck, W., Underbill, E.W., andChisholm, M.D. 1982. Structure-activity relationships in sex attractants for North American noctuid moths.J. Chem. Ecol. 8:731–754.
Tamaki, Y. (1985) Sex pheromones, pp. 145–191,in G.A. Kerkut and L.I. Gilbert (eds.). Comprehensive Insect Physiology, Biochemistry, and Pharmacology, vol. 11. Pergamon, New York.
Tamaki, Y., Kawasai, K., Yamada, H., Kashihara, T., Osaki, N., Ando, T., Yashida, S., andKakinohama, H. 1977. (Z)-11-Hexadecenal and (Z)-11-hexadecenyl acetate: Sex pheromone of the diamondback moth (Lepidoptera: Plutellidae).Appl. Entomol. Zool. 12:208–210.
Vinczer, P., Baan, G., Novak, L., andSzantay, C. 1984. A novel stereocontrolled synthesis of (Z, Z)-3, 13-octadecadien-1-yl acetate, the sex pheromone ofSynthedon species.Tetrahedron Lett. 25:2701–2704.
Vogt, R.G. 1987. The molecular basis of pheromone reception: Its role in behavior, pp. 385–431,in G.D. Prestwich and G.J. Blomquist (eds.). Pheromone Biochemistry. Academic Press, New York.
Vogt, R.G., andRiddiford, L.M. 1981. Pheromone binding and inactivation by moth antennae.Nature 293:161–163.
Vogt, R.G., andRiddiford, L.M. 1986a. Scale esterase: A pheromone-degrading enzyme from the scales of the silk mothAntheraea polyphemus.J. Chetn. Ecol. 12:469–482.
Vogt, R.G., andRiddiford, L.M. 1986b. Pheromone reception: A kinetic equilibrium, pp. 201–205,in T. Payne, R. Cardé, and J. Boeckh (eds.). Mechanisms of Perception and Orientation to Insect Olfactory Signals. Oxford University Press, Oxford.
Vogt, R.G., Riddiford, L.M., andPrestwich, G.D. 1985. Kinetic properties of a pheromone degrading enzyme: The sensillar esterase ofAntheraea polyphemus.Proc. Natl. Acad. Sci. U.S.A. 82:8827–8831.
Vogt, R.G.,Prestwich, G.D., andRiddiford, L.M. 1988. Sex pheromone receptor proteins: Visualization using a radiolabeled photoaffinity analog.J. Biol. Chem. in press.
Author information
Authors and Affiliations
Additional information
On leave from Institute of Organic Chemistry, Czechoslovak Academy of Science, Flemingovo naměstí 2, 16610, Prague, Czechoslovakia.
Rights and permissions
About this article
Cite this article
Prestwich, G.D., Streinz, L. Haloacetate analogs of pheromones: Effects on catabolism and electrophysiology inPlutella xylostella . J Chem Ecol 14, 1003–1021 (1988). https://doi.org/10.1007/BF01018789
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01018789