Abstract
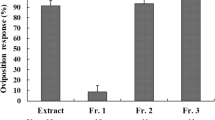
Females ofJunonia coenia (Nymphalidae), a specialist on plants that contain iridoid glycosides, were found to use aucubin and catalpol, iridoid glycosides typical of a host plant,Plantago lanceolata (Plantaginaceae), as oviposition cues. Incorporating dried ground leaf material or pure iridoid glycosides into agar disks proved to be a very effective method of testing. In no-choice tests and choice tests, females laid more eggs on disks withP. lanceolata leaf material or iridoid glycosides, compared to agar controls. There was variation among individual females in preference for disks withP. lanceolata leaf material versus disks with iridoid glycosides. Females given a choice of three different concentrations of iridoid glycoside (0.2, 0.5, 1.0%) in the agar disks and a control laid more eggs on the disk with the highest concentration of iridoid glycoside.
Similar content being viewed by others
References
Bobbitt, J.M., andSegebarth, D.P. 1969. Iridoid glycosides and similar substances, pp. 1–145,in W.I. Taylor and A.R. Battersby (eds.). Cyclopentanoid Terpene Derivatives. Marcel Dekker, New York.
Bowers, M.D. 1981. Unpalatability as a defense strategy of western checkerspot butterflies (Euphydryas).Evolution 35:367–375.
Bowers, M.D. 1983. The role of iridoid glycosides in hostplant specificity of checkerspot butterflies.J. Chem. Ecol. 9:475–493.
Bowers, M.D. 1984. Iridoid glycosides and host-plant specificity in larvae of the buckeye butterfly,Junonia coenia (Nymphalidae).J. Chem. Ecol. 10:1567–1577.
Bowers, M.D., andPuttick, G.M. 1986. Fate of ingested iridoid glycosides in lepidopteran herbivores.J. Chem. Ecol. 12:169–178.
Brower, L.P. 1984. Chemical defense in butterflies, pp. 109–134,in R.I. Vane-Wright and P.R. Ackery (eds.). The Biology of Butterflies. Academic Press, London.
Calvert, W.H. 1974. The external morphology of foretarsal receptors involved with host discrimination by the nymphalid butterfly,Chlosyne lacinia.Ann. Entomol. Soc. Am. 67:853–856.
Calvert, W.H., andHanson, F. 1983. The role of sensory structures and preoviposition behavior in oviposition by the patch butterfly,Chlosyne lacinia.Entomol. Exp. Appl. 33:179–187.
Chew, F.S. 1977. Coevolution of pierid butterflies and their cruciferous foodplants. II. The distribution of eggs on potential foodplants.Evolution 31:568–579.
Chew, F.S., andRobbins, R. 1984. Egg-laying in butterflies, pp. 65–79,in R.I. Vane-Wright and P.R. Ackery (eds.). The Biology of Butterflies. Academic Press, London.
Conover, W.J. 1980. Practical Nonparametric Statistics. John Wiley & Sons, New York.
David, W.A.L., andGardner, B.O.C. 1962. Oviposition and the hatching of the eggs ofPieris brassicae (L.) in a laboratory culture.Bull. Entomol. Res. 53:91–109.
Dethier, V.G. 1959. Egg-laying habits of Lepidoptera in relation to available food.Can. Entomol. 91:554–561.
Dethier, V.G. 1982. Mechanisms of host-plant recognition.Entomol. Exp. Appl. 31:49–56.
Duff, R., Bacon, J., Mundie, C., Farmer, V., Russell, J., andForrester, A. 1965. Catalpol and methylcatalpol: Naturally occurring glycosides inPlantago andBuddleia species.Biochem. J. 96:1–5.
El-Naggar, L.J., andBeal, J.L. 1980. Iridoids: A review.J. Nat. Prod. 43:649–707.
Feeny, P., Rosenberry, L., andCarter, M. 1983. Chemical aspects of oviposition behavior in butterflies, pp. 27–76,in S. Ahmad (ed.). Herbivorous Insects: Host-Seeking Behavior and Mechanisms. Academic Press, New York.
Fox, R. 1966. Forelegs of butterflies I. Introduction: Chemoreception.J. Res. Lepid. 3:159–172.
Harris, G.H., Jing, W., andStermitz, F.S. 1986. Iridoids and alkaloids fromCastilleja (Scro-phulariaceae) host plants forPlatyptilia pica and rhexifoline content ofP.pica. Biochem. Syst. Ecol. 14:499–506.
Honda, K. 1986. Flavanone glycosides as oviposition stimulants in a papilionid butterfly,Papilio protenor.J. Chem. Ecol. 12:1999–2010.
Hovanitz, W., andChang, V. 1964. Adult oviposition responses inPieris rapae.J. Res. Lepid. 3:159–172.
Ichinose, T., andHonda, H. 1978. Ovipositional behavior ofPapilio protenor demetrius Cramer and the factors involved in its host plants.Appl. Entomol. Zool. 13:103–114.
Ilse, D. 1937. New observations on responses to colours in egg-laying butterflies.Nature 140:544.
Jensen, S., Nielsen, B., andDahlgren, R. 1975. Iridoid compounds, their occurrence and systematic importance in the angiosperms.Bot. Not. 128:148–180.
Nishida, R. 1977. Oviposition stimulants of some papilionid butterflies contained in their host plants.Botyu-Kagaku 42:133–140.
Ohsugi, T., Nishida, R., andFukami, H. 1985. Oviposition stimulant ofPapilio xuthus, aCitrus- feeding swallowtail butterfly.Agric. Biol. Chem. 49:1897–1900.
Papaj, D.R., andRausher, M.D. 1983. Individual variation in host location by phytophagous insects, pp. 77–124.in A. Ahmad (ed.). Herbivorous Insects: Host-Seeking Behaviour and Mechanisms. Academic Press, New York.
Renwick, J.J.A. 1985. Comparative mechanisms of host selection by insects attacking pine trees and crucifers. Presentation at American Institute of Biological Sciences symposium on Chemistry and Coevolution, Gainesville, Florida.
Saxena, K.N., andGoyal, S. 1978. Host-plant relations of the citrus butterflyPapilio demoleus L.: Orientational and ovipositional responses.Entomol. Exp. Appl. 24:1–10.
Schoonhoven, L. 1972. Secondary plant substances and insects.Recent Adv. Phytochem. 5:197–224.
Scott, J. 1975. Movement ofPrecis coenia, a “pseudoterritorial” submigrant (Lepidoptera: Nymphalidae).J. Anim. Ecol. 44:843–850.
Shapiro, A.M. 1974. Butterflies of the Suisun Marsh, California.J. Res. Lepid. 13:191–206.
Singer, M.C. 1971. Evolution of food-plant preference in the butterflyEuphydryas editha.Evolution 25:383–389.
Singer, M.C. 1982. Quantification of host specificity by manipulation of Oviposition behavior in the butterflyEuphydryas editha.Oecologia 52:224–229.
Singer, M. 1983. Determinants of multiple host use by a phytophagous insect population.Evolution 37:389–403.
Sokal, R.R., andRohlf, F.J. 1969. Biometry. Freeman, San Francisco.
Stanton, M.L. 1979. The role of chemotactile stimuli in the Oviposition preference ofColias butterflies.Oecologia 39:79–91.
Stanton, M.L., andCook, R.E. 1984. Sources of intraspecific variation in the hostplant seeking behavior ofColias butterflies.Oecologia 61:265–270.
Stermitz, P.S., Gardner, D.R., Odendaal, F.J., andEhrlich, P.R. 1986.Euphydryas anicia utilization of iridoid glycosides fromCastilleja andBesseya (Scrophulariaceae) hostplants.J. Chem. Ecol. 12:1459–1468.
Tabashnik, B.E., Wheelock, H., Rainbolt, H.D., andWatt, W.B. 1981. Individual variation in Oviposition preference in the butterfly,Colias eurytheme.Oecologia 50:225–230.
Wiklund, C. 1974. Oviposition preferences inPapilio machaon in relation to the hostplants of the larvae.Entomol. Exp. Appl. 17:189–198.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pereyra1, P.C., Bowers, M.D. Iridoid glycosides as oviposition stimulants for the buckeye butterfly,Junonia coenia (Nymphalidae). J Chem Ecol 14, 917–928 (1988). https://doi.org/10.1007/BF01018783
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01018783