Abstract
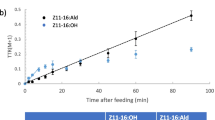
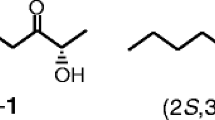
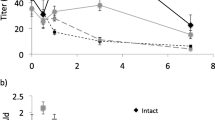
Sex pheromone biosynthesis in the codling mothCydia pomonella (Lepidoptera; Tortricidae) was studied by topical application of deuterated fatty acids in DMSO to pheromone glands. The incorporation of deuterium label into fatty acids and alcohols in the pheromone gland was monitored by gas chromatography with flame ionization detection and mass spectrometry in the selected ion monitoring mode. Dodecanol, (E)-9-dodecenol, (E,E)-8,10-dodecadienol, tetradecanol, and hexadecanol were found in gland extracts. The application of [12,12,12-2H3]dodecanoic acid resulted in labeled dodecanol, (E)-9-dodecenol, and (E,E)-8,10-dodecadienol, as well as the corresponding labeled acids. No label was incorporated into tetradecanol or hexadecanol or any acid with more than 12 carbon atoms. The application of labeled tetradecanoic or hexadecanoic acid introduced label not only into the 12-carbon alcohols, but also into tetradecanol, or tetradecanol and hexadecanol, respectively. The application of (E)-[11, 11,12,12,12,-2H5]9-dodecen-oic acid, whose facile synthesis is described, resulted in labeled (E)-9-do-decenol and (E,E)-8,10-dodecadienol. The (E,E)-8,10-dodecadienol so produced was characterized by an ion atm/z 186, equivalent to [M]+ of a dienol labeled with four deuterons. Thus, one deuterium label is lost when the labeled (E)-9-monoene is converted to the (E,E)-8,10-diene. We conclude that (E,E)-8,10-dodecadienol is synthesized by chain shortening (β-oxidation) of palmitic acid to dodecanoic acid, followed by an unusualE9 desaturation and subsequent conversion of this intermediate into the conjugated precursor, which is finally reduced to the pheromone alcohol. The evolutionary significance ofE9 desaturation being responsible for pheromone production in an Olethreutinae species is discussed.
Similar content being viewed by others
References
Arn, H., Guerin, P.M., Buser, H.R., Rauscher, S., andMani, E. 1985. Sex pheromone blend of the codling moth,Cydia pomonella: Evidence for a behavioral role of dodecan-1-ol.Experientia 41:1482–1484.
Bengtsson, M. andLiljefors, T. 1988. DMPU-An alternative to HMPT in moth sex pheromone synthesis.Synthesis (in press).
Bjostad, L.B., andRoelofs, W.L. 1981. Sex pheromone biosynthesis from radiolabeled fatty acids in the redbanded leafroller moth.J. Biol. Chem. 256:7936–7940.
Bjostad, L.B., andRoelofs, W.L. 1983. Sex pheromone biosynthesis inTrichoplusia ni: Key steps involve delta-11 desaturation and chain shortening.Science 220:1387–1389.
Bjostad, L.B., andRoelofs, W.L. 1984. Sex pheromone biosynthetic precursors inBombyx mori.Insect Biochem. 14:275–278.
Bjostad, L.B., andRoelofs, W.L. 1986. Sex pheromone biosynthesis in the red-banded leafroller moth, studied by mass labeling with stable isotopes and analysis with mass spectrometry.J. Chem. Ecol. 12:431–450.
Corey, E.J., andSmidt, G. 1979. Useful procedures for the oxidation of alcohols involving pyridinium dichromate in aprotic media.Tetrahedron Lett. 5:399–402.
Einhorn, J., Beauevais, F., Gallois, M., Descoins, C., andCausse, R. 1984. Constituants secondaires de la phéromone sexuelle du Carpocapse des Pommes,Cydia pomonella L. (Lepidoptera, Tortricidae).C.R. Acad. Sci. Paris Ser. III 299:773–778.
Folch, J., Lees, M., andSloane, S.G.H. 1957. A simple method for the isolation and purification of total lipides from animal tissue.J. Biol. Chem. 226:497–509.
Houx, N.W.H., Voerman, S., andJongen, W.M.F. 1974. Purification and analysis of synthetic insect sex attractants by liquid chromatography on a silver-loaded resin.J. Chromatogr. 96:25–32.
Löfstedt, C., andOdham, G. 1984. Analysis of moth pheromone acetates by selected ion monitoring using electron impact and chemical ionization mass spectrometry.Biomed. Mass Spectrom. 11:106–113.
Löfstedt, C., andRoelofs, W.L. 1985. Sex pheromone precursors in two primitive New Zealand tortricid moth species.Insect Biochem. 15:729–734.
Löfstedt, C., Elmfors, A., Sjögren, M., andWijk, E. 1986a. Confirmation of sex pheromone biosynthesis from [16-D3]palmitic acid in the turnip moth.Experientia 42:1059–1061.
Löfstedt, C., Löfqvist, J., Lanne, B.S., Van Der Pers, J.N.C., andHansson, B.S. 1986b. Pheromone dialects in European turnip mothsAgrotis segetum.Oikos 46:250–257.
Roelofs, W.L., andBjostad, L.B. 1984. Biosynthesis of lepidopteran pheromones.Bioorg. Chem. 12:279–298.
Roelofs, W.L., andBrown, R.L. 1982. Pheromones and evolutionary relationships of Tortricidae. 1983.Annu. Rev. Ecol. Syst. 13:395–422.
Roelofs, W.L., Comeau, A., Hill, A., andMilicevic, G. 1971. Sex attractant of the codling moth: Characterization with electroantennogram technique.Science 174:297–299.
Taber, D. 1982. TLC mesh column chromatography.J. Org. Chem. 47:1351–1352.
Warthen, J.D., Jr., andJacobson, M. 1973. Insect sex attractants; XIV. All-(trans-alkenol acetates via sodium-liquid ammonia reduction.Synthesis 10:616–617.
Yamaoka, R., Taniguchi, Y., andHayashiya, K. 1984. Bombykol biosynthesis from deuterium-labeled (Z)-11-hexadecenoic acid.Experientia 40:80–81.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Löfstedt, C., Bengtsson, M. Sex pheromone biosynthesis of (E,E)-8,10-dodecadienol in codling mothCydia pomonella involvesE9 desaturation. J Chem Ecol 14, 903–915 (1988). https://doi.org/10.1007/BF01018782
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01018782