Abstract
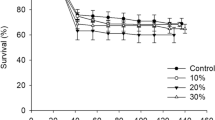
The individual and interactive effects of dietary protein and juglone on larval performance and midgut detoxification enxymes were investigated for the gypsy moth,Lymantria dispar. The experimental design was a 2 × 3 factorial, with two levels of protein and three levels of juglone. We monitored survival/development rates from egg hatch to pupation and conducted fourth-instar feeding trials for determination of nutritional indices. Enzyme solutions were prepared from midguts of fifth instars and assayed for polysubstrate monooxygenase, esterase, quinone reductase, and glutathione transferase activities. Results showed that low protein levels prolonged development times, increased consumption rates, and reduced pupal weights. Juglone markedly reduced survival, growth, and consumption rates, increased development times, and reduced pupal weights. The interaction between protein and juglone influenced larval digestion efficiencies and female pupal weights. Polysubstrate monooxygenase activities were unaffected by diet, whereas esterase activities increased in response to both low dietary protein and presence of juglone. Low protein levels increased soluble quinone reductase activities but decreased glutathione transferase activities. Glutathione transferase activities were lowest in larvae fed low-protein, high-juglone diets and may have contributed to the especially poor performance of larvae on those diets. Quinone reductase and glutathione transferase are the systems of importance in detoxification of juglone, and moderate to low activities of these enzymes may explain why gypsy moths perform poorly on members of the Juglandaceae.
Similar content being viewed by others
References
Ahmad, S., andForgash, A.J. 1973. NADPH oxidation by microsomal preparations of gypsy moth larval tissues.Insect Biochem. 3:263–273.
Ahmad, S., andForgash, A.J. 1975. NADPH-cytochrome-c reductase: Changes in specific activity in gypsy moth larvae.J. Insect Physiol. 21:85–88.
Ahmad, S., andForgash, A.J. 1978. Gypsy moth mixed-function oxidases: Gut enzyme levels increased by rearing on a wheat germ diet.Ann. Entomol. Soc. Am. 71:449–452.
Ahmad, S., Brausten, L.B., Mullin, C.A., andYu, S.J. 1986. Enzymes involved in the metabolism of plant allelochemicals, pp. 73–151,in L.B. Brattsten and S. Ahmad (eds.). Molecular Aspects of Insect-Plant Associations. Plenum Press, New York.
Berenbaum, M., andNeal, J.J. 1985. Synergism between myristicin and xanthotoxin, a naturally cooccurring plant toxicant.J. Chem. Ecol. 11:1349–1358.
Boyd, J.N., andCampbell, T.C. 1983. Impact of nutrition on detoxication, pp. 287–306,in J. Caldwell and W.B. Jakoby (eds.). Biological Basis of Detoxication. Academic Press, New York.
Brattsten, L.B. 1987. Metabolic insecticide defenses in the boll weevil compared to those in a resistance-prone species.Pestic. Biochem. Physiol. 27:1–12.
Battsten, L.B., Price, S.L., andGunderson, C.A. 1980. Microsomal oxidases in midgut and fatbody tissues of a broadly herbivorous insect larva,Spodoptera eridania Cramer (Noctuidae).Comp. Biochem. Physiol. 66C:231–237.
Broadway, R.M., andDuffey, S.S. 1988. The effect of plant protein quality on insect digestive physiology and the toxicity of plant proteinase inhibitors.J. Insect Physiol. 34:1111–1117.
Cohen, E. 1986. Glutathione-S-transferase activity and its induction in several strains ofTribolium castaneum.Entomol. Exp. Appl. 41:39–44.
Dauterman, W.C. 1980. Physiological factors affecting metabolism of xenobiotics, pp. 133–142,in E. Hodgson and F.E. Guthrie (eds.). Introduction to Biochemical Toxicology. Elsevier, New York.
Gilbert, B.L., Baker, J.E., andNorris, D.M. 1967. Juglone (5-hydroxy-l,4-naphthoquinone) fromCarya ovata, a deterrent to feeding byScolytus multistriatus.J. Insect Physiol. 13:1453–1459.
Gunderson, C.A., Brattsten, L.B., andFleming, J.T. 1986. Microsomal oxidase and glutathione transferase as factors influencing the effects of pulegone in southern and fall armyworm larvae.Pestic. Biochem. Physiol. 26:238–249.
Hedin, P.A., Langhans, V.E., andGraves, C.H., Jr. 1979. Identification of juglone in pecan as a possible factor of resistance toFusicladium effusum.J. Agric. Food Chem. 27:92–94.
Hedin, P.A., Collum, D.H., Langhans, V.E., andGraves, C.H. 1980. Distribution of juglone and related compounds in pecan and their effect onFusicladium effusum.J. Agric. Food Chem. 28:340–342.
Johnson, N.D., andBentley, B.L. 1988. Effects of dietary protein and lupine alkaloids on growth and survivorship ofSpodoptera eridania.J. Chem. Ecol. 14:1391–1403.
Lechowicz, M.J., andMauffette, Y. 1986. Host preferences of the gypsy moth in eastern North American versus European forests.Rev. D'entomol. Queb. 31:43–51.
Lincoln, D.E., Newton, T.S., Ehrlich, P.R., andWilliams, K.S. 1982. Coevolution of the checkerspot butterflyEuphydryas chalcedona and its larval food plantDiplacus aurantiacus: Larval response to protein and leaf resin.Oecologia 52:216–223.
Lindroth, R.L. 1989a. Chemical ecology of the luna moth: Effects of host plant on detoxification enzyme activity.J. Chem. Ecol. 15:2019–2029.
Lindroth, R.L. 1989b. Host plant alteration of detoxication enzyme activity inPapilio glaucus glaucus.Entomol. Exp. Appl. 50:29–35.
Margoliash, E., andFrohwirt, N. 1959. Spectrum of horse-heart cytochromec.Biochem. J. 71:570–572.
Mattson, W.J., Jr. 1980. Herbivory in relation to plant nitrogen content.Annu. Rev. Ecol. Syst. 11:119–161.
Norris, D.M. 1988.Periplaneta americana perception of phytochemical naphthoquinones as allelochemicals.J. Chem. Ecol. 14:1807–1819.
Odell, T.M., Butt, C.A., andBridgeforth, A.W. 1985.Lymantria dispar, pp. 355–367,in P Singh and R.F. Moore (eds.). Handbook of Insect Rearing, Vol. 2. Elsevier, New York.
Parke, D.V., andIoannides, C. 1981. The role of nutrition in toxicology.Annu. Rev. Nutr. 1:207–234.
Ponder, F., Jr. 1987. Allelopathic interference of black walnut trees with nitrogen-fixing plants in mixed plantings, pp. 195–204,in G.R. Waller (ed.). Allelochemicals. Role in Agriculture and Forestry. American Chemical Society, Washington, D.C.
Rossiter, M.C. 1987. Genotypic and phenotypic variation in diet breadth in a generalist herbivore.Evol. Ecol. 1:272–282.
Schacterle, G.R., andPollack, R.L. 1973. A simplified method for quantitative assay of small amounts of protein in biologic material.Anal. Biochem. 51:654–655.
Scriber, J.M., andSlansky, F., Jr. 1981. The nutritional ecology of immature insects.Annu. Rev. Entomol. 26:183–211.
Segel, I.H. 1976. Biochemical Calculations, 2nd ed. John Wiley & Sons, New York.
Sheppard, C.A., andFriedman, S. 1989. Endogenous and induced monooxygenase activity in gypsy moth larvae feeding on natural and artificial diets.Arch. Insect Biochem. Physiol. 10:47–56.
Slansky, F., Jr., andScriber, J.M. 1985. Food consumption and utilization, pp. 87–163,in G.A. Kerkut and L.I. Gilbert (eds.). Comprehensive Insect physiology, Biochemistry and Pharmacology, Vol. 4. Pergamon Press, New York.
Wadleigh, R.W., andYu, S.J. 1987. Glutathione transferase activity of fall armyworm larvae toward α, β-unsaturated carbonyl allelochemicals and its induction by allelochemicals.Insect Biochem. 17:759–764.
White, T.C.R. 1984. The abundance of invertebrate herbivores in relation to the availability of nitrogen in stressed food plants.Oecologia 63:90–105.
Yu, S.J. 1987. Quinone reductase of phytophagous insects and its induction by allelochemicals.Comp. Biochem. Physiol. 87B:621–624.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lindroth, R.L., Anson, B.D. & Weisbrod, A.V. Effects of protein and juglone on gypsy moths: Growth performance and detoxification enzyme activity. J Chem Ecol 16, 2533–2547 (1990). https://doi.org/10.1007/BF01017476
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01017476