Summary
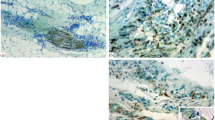
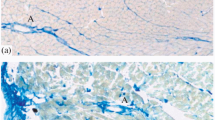
In this communication, the results of an enzyme histochemical study on the working myocardial fibres and Purkinje fibres of the atrioventricular conducting system of the human heart under ischaemic and inflammatory conditions are presented. The material was selected from patients showing changes which could be classified in three major groups: (1) early changes due to acute ischaemia either in the myocardial fibres or in the conducting system or in both; (2) chronic ischaemic changes due to cardiovascular insufficiency, such as old infarction, or coronary arteriosclerosis or both; and (3) inflammatory conditions such as myocarditis.
The activity and location of about 20 enzymes that play a role in the aerobic and anaerobic pathways of energy metabolism were examined. The activity and location of some hydrolytic enzymes and the glycogen and lipid content were also studied.
The most important findings were an obvious depletion of the glycogen reserves under acute ischaemic changes in both types of fibre. This was associated with a transient or permanent reduction in activity of many enzymes that play a role in aerobic and anaerobic metabolism. Further, there was an instantaneous and persistent increase in the activity of the NADPH-regenerating enzymes of the pentose phosphate pathway and of glyceraldehyde-3-phosphate dehydrogenase, the rate-limiting enzyme of glycolysis under ischaemic conditions. Chronic ischaemic changes were characterized by a gradual long-term increase in the activity of many anaerobic glycolytic enzymes. Moreover, there was an absence of activity of acetylcholine esterase immediately after the onset of infarction in the fibres of the conducting system. Lastly, a slight increase in lipid content was found in the hypertrophic chronic ischaemic fibres and in old infarcted areas. Cardiac fibres in inflamed areas showed a marked increased activity of the pentose phosphate shunt enzymes and a less pronounced increased activity of most anaerobic and hydrolytic enzymes. In contrast to the cardiac fibres in infarcted areas, the fibres in inflamed areas did not reveal a decrease or absence of activity of aerobic enzymes such as succinate dehydrogenase.
Similar content being viewed by others
References
Alcine, E., Lageron, A. &Wegmann, R. (1965) Étude histoenzymologique du métabolism glucidique du faisceau de His à différent niveaux et du myocarde ventriculaire, chez le rat.Ann. Histochim. 10, 127–44.
Anderson, K. R., Popple, A., Parker, D. J., Sayer, R., Trickey, R. J. &Davies, M. J. (1979) An experimental assessment of macroscopic enzyme techniques for the autopsy demonstration of myocardial infarction.J. Path. 217, 93–110.
Bajusz, E. &Jasmin, G. (1964a) Histochemical studies on the myocardium following experimental interference with coronary circulation in the rat. I. Occlusion of coronary artery.Acta histochem. 18, 222–37.
Bajusz, E. &Jasmin, G. (1964b) Histochemical studies on the myocardium following experimental interference with coronary circulation in the rat. II. Occlusion of coronary veins.Acta histochem. 18, 238–50.
Barka, M. D. &Anderson, P. J. (1963)Histochemistry, Theory, Practice and Bibliography. New York: Harper and Row.
Burstone, M. S. (1962)Enzyme Histochemistry. New York: Academic Press.
Christie, K. N. &Stoward, P. J. (1978) Endogenous peroxidase in mast cells localized with a semi-permeable membrane technique.Histochem. J. 10, 425–33.
Cornblatt, M., Randle, P. J., Parmeggiani, A. &Morgan, H. E. (1963) Regulation of glycogenolysis in muscle: Effects of glucagon and anoxia on lactate production, glycogen content and phosphorylase activity in the perfused isolated rat heart.J. biol. Chem. 238, 1592–7.
Dreyfus, J. C., Schapira, G., Resnais, J. &Scebat, L. (1960) La créatinkinase sérique dans le diagnostic de l'infarctes myocardique.Rev. franç. Etud. clin. Biol. 5, 386–7.
Elias, E. A., Elias, R. A. &Van Der Baan, R. (1981) Metastasizing carcinoma of Bartholin's gland. An enzyme histochemical and cytological study.Acta histochem. 69, 31–9.
Elias, E. A., Elias, R. A., Bijlsma, P. J. &Tazelaar, D. J. (1980b) The enzyme histochemistry of metastasizing basal cell carcinomaJ. Path. 131, 235–42.
Elias, E. A. &Meijer, A. E. F. H. (1981) The increase in activity of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in skeletal muscles of rats after subcutaneous administration ofN,N′-dimethyl-para-phenylene-diamine.Histochemistry 71, 543–58.
Elias, E. A., De Vries, G. P., Elias, R. A., Tigges, A. J., &Meijer, A. E. F. H. (1980a) Enzyme histochemical studies on the conducting system of the human heart.Histochem. J. 12, 577–89.
Evans, J. R. (1964) Cellular transport of long chain fatty acids.Can. J. Biochem. 42, 955–68.
Fine, G., Morales, A. &Scerpella, J. R. (1966) Experimental myocardial infarction. A. histochemical study.Archs Path. 82, 4–8.
Freedland, R. A. (1968) Considerations in the estimation of enzyme half-lives in higher animals by rates of changes in activities.Life Sci. 7, 499–503.
Glenner, G. G., Burtner, H. J. &Brown, G. W. (1957) The histochemical demonstration of monoamine oxidase activity by tetrazolium salts.J. Histochem. Cytochem. 5, 591–600.
Goldberg, A. &St John, A. C. (1976) Intracellular protein degradation in mammalian and bacterial cells. Part. 2.Ann. Rev. Biochem. 45, 747–803.
Gould, S. E. (1960)Pathology of the Heart. 2nd edn., pp. 599–606. Springfield, Illinois: Thomas.
Jestädt, R. &Sandritter, W. (1959) Erfahrungen mit der TTC-(Triphenyl-tetrazoliumchlorid) Reaktion bei der pathologisch-anatomischen Diagnose des frischen Herzinfarktes.Z. Kreisl. Forsch. 48, 802–9.
Karnovsky, M. J. &Roots, L. (1964) A ‘direct-coloring’ thiocholine method for choline esterase.J. Histochem. Cytochem. 12, 219–21.
Krebs, H. A. (1972) Some aspects of the regulation of fuel supply in omnivorous animals. InEnzyme Regulation, Vol. 10 (edited byWeber, G.), pp. 397–420. Oxford: Pergamon Press.
Kubler, W. &Spieckermann, P. G. (1970) Regulation of glycolysis in the ischaemic and anoxic myocardium.J. molec. cell. Cardiol. 1, 351–77.
Lodja, Z., Gossrau, R. &Schiebler, T. H. (1979)Enzyme Histochemistry. Berlin, Heidelberg, New York: Springer-Verlag.
Mallory, G. K., White, P. D. &Salcedo-Salgar, J. (1939) The speed of healing of myocardial infarcation.Am. Heart. J. 18, 647–71.
Meijer, A. E. F. H. (1968) Improved histochemical method for the demonstration of the activity of α-glucan phosphorylase 1. The use of glucosyl acceptor dextran.Histochemie 12, 244–52.
Meijer, A. E. F. H. (1970) Histochemical method for the demonstration of myosin adenosine triphosphatase in muscle tissue.Histochemie 22, 51–8.
Meijer, A. E. F. H. (1972) Semipermeable membranes for improving the histochemical demonstration of enzyme activities in tissue sections. I. Acid phosphatase.Histochemie 30, 31–9.
Meijer, A. E. F. H. (1973) Semipermeable membranes for improving the histochemical demonstration of enzyme activities in tissue sections. III. Lactate dehydrogenase.Histochemie 35, 165–72.
Meijer, A. E. F. H., Benson, D. &Scholte, H. R. (1977a) The influence of freezing and freeze-drying of tissue specimens on enzyme activity.Histochemistry 51, 297–303.
Meijer, A. E. F. H. &Elias, E. A. (1977) Die Aktivität der Glucose-6-Phosphat-Dehydrogenase and 6-Phosphogluconat-Dehydrogenase in Skelettmuskelgewebe von Patienten mit Muskelkrankheiten.Acta histochemica. Suppl.18, 169–75.
Meijer, A. E. F. H., Elias, E. A. &Vloedman, A. H. T. (1977b) The value of enzyme histochemical techniques in classifying fibre types of human skeletal muscle. III. Human skeletal muscles with inherited or acquired disease of the neuromuscular system.Histochemie 53, 97–105.
Meijer, A. E. F. H. &Stegehuis, F. (1980) Histochemical technique for the demonstration of phosphofructokinase activity in heart and skeletal muscles.Histochemistry 66, 75–81.
Meijer, A. E. F. H. &Vloedman, A. H. T. (1973) Semipermeable membranes for improving the histochemical demonstration of enzyme activities in tissue sections. II. Non specific esterase and β-glucuronidase.Histochemie 34, 127–34.
Meijer, A. E. F. H. &Vloedman, A. H. T. (1980) The histochemical characterization of the coupling state of skeletal muscle mitochondria.Histochemistry 69, 217–32.
Meijer, A. E. F. H. &De Vries, G. P. (1974) Semipermeable membranes for improving the histochemical demonstration of enzyme activities in tissue sections. IV. Glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase (decarboxylating).Histochemistry 40, 349–59.
Meijer, A. E. F. H. &De Vries, G. P. (1975) Semipermeable membranes for improving the histochemical demonstration of enzyme activities in tissue sections. V. Isocitrate: NADP+ oxidoreductase (decarboxylating).Histochemistry 43, 225–36.
Meijer, A. E. F. H. &De Vries, G. P. (1978) Enzyme histochemical studies on the Purkinje fibres of the atrioventricular system of the bovine and porcine hearts.Histochem. J. 10, 399–408.
Morales, A. R. &Fine, G. (1966) Early human myocardial infarction. A histochemical study.Archs Path. 82, 9–14.
Morgan, H. E., Henderson, M. J., Regen, D. M. &Park, C. R. (1959) Regulation of glucose uptake in heart muscle from normal and alloxan-diabetic rats. Effects of insulin, growth hormone, cortisone and anoxia.Ann. N.Y. Acad. Sci. 82, 387–402.
Nachlas, M., Tsou, K. C., De Souza, E., Cheng, C-S. &Seligman, A. M. (1957) The cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole.J. Histochem. Cytochem. 5, 420–36.
Nachlas, M. M. &Shnitka, T. K. (1963) Macroscopic identification of early myocardial infarcts by alterations in dehydrogenase activity.Am. J. Path. 42, 379–405.
Opie, L. H. (1976) II. Metabolic regulation in ischemia and hypoxia. Effects of regional ischemia on metabolism of glucose and fatty acids. Suppl. 1,Circ. Res. 38, 53–67.
Otsuka, N., Hara, T. &Okamoto, H. (1967) Histotopochemische Untersuchungen am Reizleitungssystem des Hundeherzens.Histochemie 10, 66–73.
Rovetto, M. J., Lamberton, W. F. &Neely, J. R. (1975) Mechanisms of glycolytic inhibition in ischemic rat hearts.Circ. Res. 37, 742–51.
Schiebler, T. H. (1961) Histochemische Untersuchungen am Reizleitungssystem tierischer Herzen.Naturwissenschaften 14, 502–3.
Schiebler, T. H. (1963) Über den histochemischen Nachweis von Atmungsfermenten im Reizleitungssystem.Anat. Anz. 111, 103–12.
Schiebler, T. H., Stark, M. &Caesar, R. (1956) Die Stoffwechselsituation des Reizleitungssystems.Klin. Wschr. 34, 181–3.
Shnitka, T. K. &Nachlas, M. M. (1963) Histochemical alterations in ischaemic heart muscle and early myocardial infarction.Am. J. Path. 42, 507–27.
Snijder, J. &Meijer, A. E. F. H. (1970) Enzyme histochemical studies on the Purkinje fibres of canine, bovine and porcine hearts.Histochem. J. 2, 395–409.
Vries, G. P. De &Meijer, A. E. F. H. (1976) Semipermeable membranes for improving the histochemical demonstration of enzyme activities in tissue sections. VI.d-glucose-6-phosphate isomerase and phosphoglucomutase.Histochemistry 50, 1–8.
Vries, G. P. De, Tigges, A. J. &Meijer, A. E. F. H. (1980) The histochemical demonstration of glyceraldehyde phosphate dehydrogenase activity with a semipermeable membrane technique.Histochem. J. 12, 119–22.
Wachstein, M. &Meisel, E. (1955) Succinic dehydrogenase activity in myocardial infarction and in induced myocardial necrosis.Am. J. Path. 31, 353–65.
Wildenthal, K. (1975) Lysosomal and lyosomal enzymes in the heart. InLysosomes in Biology and Pathology, Vol. 4 pp. 167–190. (edited byDingle, J. T. andDean, R. T.), Amsterdam, Oxford: North Holland.
Williamson, J. R. (1966) Glycolytic control mechanisms II. Kinetics of intermediate changes during the aerobic-anoxic transition in perfused rat hearts.J. biol. Chem. 241, 5026–36.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Elias, E.A., Elias, R.A., De Vries, G.P. et al. Early and late changes in the metabolic pattern of the working myocardial fibres and Purkinje fibres of the human heart under ischaemic and inflammatory conditions: An enzyme histochemical study. Histochem J 14, 445–459 (1982). https://doi.org/10.1007/BF01011856
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01011856