Summary
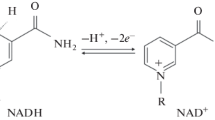
The correct localization of oxidative enzymes using cytochemical tetrazolium methods, in which low molecular weight electron carriers such as NAD(P)H and reduced phenazine methosulphate (PMSH) are used, can be endangered by the escape of the reduced intermediates before they react to form the insoluble formazan at the true enzyme-containing sites. To investigate this phenomenon, the glucose-6-phosphate dehydrogenase reaction was studied in fixed erythrocytes which, because of their microscopic dimensions, are well-suited for studying the loss of intermediates.
A mixture of active and heat-inactivated fixed erythrocytes was incubated in a PMS-supplemented medium for glucose-6-phosphate dehydrogenase. The cytophotometric histograms showed that the final formazan precipitate was equally distributed over both active and inactivated cells. When bovine serum albumin was added to the medium, all the formazan was found to be bound to this protein and the erythrocytes remained essentially unstained. The false localization in this system could be explained by an unfavourable balance between the capture of electrons carried by NADPH within the erythrocyte and the diffusion of NADPH out of the erythrocyte. The rate constant of NADPH oxidation was determined, as was also the diffusion constant of NADPH in a protein matrix. Substituting the data obtained into formulae derived from the enzyme cytochemical localization theory of Holt & O'Sullivan (1958), it was calculated that the capture reaction was highly deficient and, theoretically, less than 1% of the total amount of formazan produced was localized within the erythrocyte which explains the false localization observed. The importance of these findings for the cytochemical demonstration of NAD(P)+-dependent dehydrogenases in cells and electropherograms is briefly discussed.
Similar content being viewed by others
References
Altman, F. P. (1972) Quantitative dehydrogenase cytochemistry with special reference to the pentose shunt dehydrogenases.Prog. Histochem. Cytochem. 4, 225–73.
Altman, F. P. (1976) Tetrazolium salts and formazans.Prog. Histochem. Cytochem. 9, 1–56.
Andersen, H. &Hoyer, P. E. (1974) Simplified control experiments in the histochemical study of coenzyme-linked dehydrogenases.Histochemistry 38, 71–83.
Aragon, J. J., Felin, J. E., Frenkel, R. A. &Sols, A. (1980) Permeabilization of animal cells for kinetic studies of intracellular enzymes:In situ behavior of the glycolytic enzymes of erythrocytes.Proc. natn. Acad. Sci. U. S. A. 77, 6324–8.
Beutler, E. &Morrison, M. (1967) Localization and characteristics of hexose-6-phosphate dehydrogenase (glucose dehydrogenase).J. biol. Chem. 242, 5289.
Butcher, R. G., Dawson, A. L., Knaab, S. A. &Gahan, P. B. (1980) Dehydrogenase activity and loss of formazan from tissue sections.Histochem. J. 12, 591–8.
Cornelisse, C. J., Hermens, W. Th., Tjok, J. M., Duijndam, W. A. L. &Van Duijn, P. (1976) A theoretical study of concentration profiles of primary cytochemical enzyme reaction products in membrane-bound cell organelles and its application to lysosomal acid phosphatase.Histochem. J. 8, 609–24.
Cornelisse, C. J. &Van Duijn, P. (1973) A new method for the investigation of the capture reaction in phosphatase cytochemistry. II. Theoretical and experimental study of phosphate diffusion from thin polyacrylamide films.J. Histochem. Cytochem. 21, 614–22.
De Jong, A. S. H. (1982) Mechanisms of metal-salt methods in enzyme cytochemistry with special reference to acid phosphatase.Histochem. J. 14, 1–33.
De Jong, A. S. H., Hak, T. J., Tjokjoe, M. &Van Duijn, P. (1978) Enzyme layers on glass as a new model for the quantitative study of capture reactions on enzyme cytochemistry, with special reference to acid phosphatase.Histochemistry 57, 273–84.
De Jong, A. S. H. &Van Der Ploeg, M. (1977) Cytochemical and biochemical latency of alkaline phosphatase in granules of neutrophilic leucocytes.J. Histochem. Cytochem. 25, 1184–6.
De Jong, A. S. H., Van Duijn, P. &Daems, W. Th. (1976) Cytochemical model system for microsomal rat liver glucose-6-phosphatase.J. Histochem. Cytochem. 24, 643–51.
Dewey, M. M. &Conklin, J. L. (1960) Starch gel electrophoresis of lactic dehydrogenase from rat kidney.Proc. Soc. exp. Biol. 105, 492–4.
Farber, E. &Bueding, E. (1956) Histochemical localization of specific oxidative enzymes. V. The dissociation of succinic dehydrogenase from carriers by lipase and the specific histochemical localization of dehydrogenase with phenazine methosulphate.J. Histochem. Cytochem. 4, 357–62.
Farber, E. &Louviere, C. D. (1956) Histochemical localization of specific oxidative enzymes. IV. Soluble oxidation reduction dyes as aids in the histochemical localization of oxidative enzymes with tetrazolium salts.J. Histochem. Cytochem. 4, 347–56.
Gillet, R. &Gull, K. (1972) Glutaraldehyde-its purity and stability.Histochemie 30, 162–7.
Holt, S. J. &O'Sullivan, D. G. (1958) Studies in enzyme cytochemistry. I. The principles of cytochemical staining methods.Proc. R. Soc., B 148, 465–80.
Kugler, P. (1979) A gel-sandwich technique for the qualitative and quantitative determination of dehydrogenases in the enzyme cytochemistry. I. Development of the new method on the example of LDH (E.C. 1.1.1.27).Histochemistry 60, 265–93.
Kugler, P. &Wrobel, K. H. (1978) Meldola blue: A new electron carrier for the histochemical demonstration of dehydrogenases (SDH, LDH, G6PDH).Histochemistry 59, 97–107.
Morselt, A. F. W. (1978) Application and possibilities of cytophotometry for red cell hematology.Prog. Histochem. Cytochem. 11, 1–42.
Nachlas, M. M., Kamarkar, S. S. &Seligman, A. M. (1960) The determination of lactic dehydrogenase with a tetrazolium salt.Analyt. Biochem. 1, 317–26.
Pearse, A. G. E. (1972)Histochemistry, Theoretical and Applied. Vol. 2, 3rd edn. Edinburgh, London: Churchill-Livingstone.
Prosperi, E. &Raap, A. K. (1982) Substrate protection during the fixation of glucuronidase: cytochemical model system studies.Histochem. J. 14, 689–91.
Raap, A. K. &Van Duijn, P. (1981) Enzyme incorporated erythrocyte ghosts: A new model system for quantitative enzyme cytochemistry.J. Histochem. Cytochem. 29, 1418–24.
Rieder, H., Teutsch, H. F. &Sasse, D. (1978) NADP dependent dehydrogenases in rat liver parenchyma. I. Methodological studies on the qualitative histochemistry of G6PDH, malic enzyme and ICDH.Histochemistry 56, 283–98.
Rozenszjan, L. &Shoham, D. (1967) The demonstration of dehydrogenases and diaphorases in cells of peripheral blood and bone marrow.Blood 29, 737–46.
Singer, T. P. &Kearny, E. B. (1954) Determination of succinic dehydrogenase activity. InMethods of Biochemical Analysis (edited byGlick, D.), Vol. IV, pp. 307–33. New York: Interscience Publishers.
Tanahashi, K. &Horni, S. H. (1980) Immunohistochemical localization of hexose-6-phosphate dehydrogenase in various organs of the rat.J. Histochem. Cytochem. 28, 1175–82.
Van der Ploeg, M., Van Den Broek, K., Smeulders, A. W. M., Vossepoel, A. M. &Van Duijn, P. (1977) HIDACSYS: computer programs for interactive scanning cytophotometry.Histochemistry 54, 273–87.
Van Der Ploeg, M. &Van Duijn, P. (1964) 5,6-dihydroxyindole as a substrate in a histochemical peroxidase reaction.Jl. R. microsc. Soc. 83, 415–23.
Van Der Ploeg, M. &Van Duijn, P. (1968) Cytophotometric determination of alkaline phosphatase activity in individual neutrophilic leucocytes with a biochemically calibrated model system.J. Histochem. Cytochem. 16, 693–706.
Van Duijn, P., Pascoe, E. &Van Der Ploeg, M. (1967). Theoretical and experimental aspects of enzyme determination in a cytochemical model system of polyacrylamide films containing alkaline phosphatase.J. Histochem. Cytochem. 15, 631–45.
Van Duijn, P. (1973) Fundamental aspects of enzyme cytochemistry. InElectron Microscopy and Cytochemistry (edited byWisse, E., Daems, W. Th., Molenaar, I. andVan Duijn, P.), pp. 3–23. Amsterdam: North-Holland.
Van Noorden, C. J. F. &Tas, J. (1980) Quantitative aspects of the cytochemical demonstration of glucose-6-phosphate dehydrogenase with tetranitroBT studied in a model system of polyacrylamide films.Histochem. J. 12, 669–85.
Van Noorden, C. J. F. &Tas, J. (1982) The role of exogeneous electron carriers in NAD(P)-dependent dehydrogenase cytochemistry studiedin vitro and with a model system of polyacrylamide films.J. Histochem. Cytochem. 30, 12–20.
Zimmerman, N. &Geyer, G. (1981) A polyacrylamide gel method for the cytochemical demonstration of glucose-6-phosphate dehydrogenase activity in mouse sperm.Acta histochem. 68, 227–30.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Raap, A.K., Van Hoof, G.R.M. & Van Duijn, P. Studies on the phenazine methosulphate-tetrazolium salt capture reaction in NAD(P)+-dependent dehydrogenase cytochemistry. I. Localization artefacts caused by the escape of reduced co-enzyme during cytochemical reactions for NAD(P)+-dependent dehydrogenases. Histochem J 15, 861–879 (1983). https://doi.org/10.1007/BF01011826
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01011826