Abstract
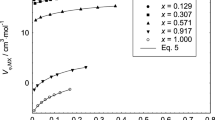
A unified treatment of liquid junction potentials and membrane potentials which accounts for both ionic and solvent transfers at homoionic junctions between ultra concentrated electrolyte solutions, also in terms of the primary hydration parameters and the Stokes-Robinson hydration theory, is described. Application to the determination of cation transference numbers, τ+, water transference numbers, τw, and primary hydration numbers,h, is described as a rational scheme for characterization of concentrated electrolytes as possible new salt bridges for the minimization of liquid junction potentials in electroanalysis. Examples of application of this scheme are presented based on multiple regression analysis of electromotive force measurements of such homoionic concentration cell as Ir | Cl2 | HCl (m 2) ‖ HCl (m 1) | Cl2 | Ir and Hg | Hg2SO4 | Li2SO4 (m 2) ‖ Li2SO4 (m 1) | Hg2SO4 | Hg, with fixedm 1 molality and variedm 2 molality. Based on the electromotive force of analogous homoionic transference cells but with interposed membranes, application of the present procedure can be extended to the determination of ion and solvent transport parameters, notably the degree of permselectivity, of membranes for use either as selective sensors in electroanalysis or selective separators in industrial electrochemistry.
Similar content being viewed by others
References
D. J. G. Ives and G. J. Janz, ‘Reference Electrodes —Theory and Practice’, Academic Press, New York (1961) p. 51.
J. A. Christiansen and M. Pourbaix,Compt. Rend. Conf. Union Int. Chim. Pure Appl., 17o Conférence, Stockholm (1953) p. 83.
R. G. Bates, ‘Determination of pH — Theory and Practice’, 2nd edn., Wiley, New York (1973), pp. 12–14, 38.
Ref. D. J. G. Ives and G. J. Janz, ‘Reference Electrodes —Theory and Practice’, Academic Press, New York (1961) pp. 12–14, 26–31.
R. M. Hawthorne,J. Chem. Educ. 50 (1973) 282.
G. Scatchard,J. Amer. Chem. Soc. 75 (1953) 2883.
M. Spiro,J. Chem. Educ. 33 (1956) 464.
D. Feakins,J. Chem. Soc. (1961) 5308.
D. Feakins and J. W. Lorimer,Chem. Comm. (1971) 646.
C. L. de Ligny, A. G. Remijnse and N. G. van der Veen,J. Electroanal. Chem. Interf. Elecrochem. 45 (1973) 488.
R. H. Stokes and R. A. Robinson,J. Amer. Chem. Soc. 70 (1948) 1870.
H. S. Harned and B. B. Owen, ‘The Physical Chemistry of Electrolytic Solutions’, 3rd edn., Reinhold, New York (1958) pp. 49, 50, 53, 59–70, 165–8, 508–25.
R. A. Robinson and R. H. Stokes, ‘Electrolyte Solutions’, 2nd rev. edn., Butterworths, London (1965) pp. 229–252.
R. H. Stokes and R. A. Robinson,J Solution Chem. 2 (1973) 173.
S. Rondinini, P. Longhi, P. R. Mussini and T. Mussini,Proc. 10th I.F.C.C. Symposium on Methodology and Clinical Applications of Ion Selective Electrodes. vol. 10, Utrecht (1989) p. 139.
K. Camman, ‘Working with Ion-Selective Electrodes’, Springer Verlag, Berlin (1979) pp. 35–48.
T. Mussini,J. Chem. Education 65 (1988) 242; and P. Longhi, P. R. Mussini and S. Rondinini,Ann. Chim. (Rome) 77 (1987) 533.
A. Davies and B. Steel,J. Solution Chem. 13 (1984) 349.
F. King and M. Spiro,J. Solution. Chem. 12 (1983) 65.
H. S. Harned and E. C. Dreby,J. Amer. Chem. Soc. 61 (1939) 3113.
S. Lengyel, J. Giber and J. Tamàs,Acta Chem. Acad. Sci. Hung. 32 (1962) 429.
S. Rondinini, A. Cavadore, P. Longhi and T. Mussini,J. Chem. Thermodynamics 20 (1988) 711.
R. H. Stokes,J. Amer. Chem. Soc. 76 (1954) 1988.
Ref. R. A. Robinson and R. H. Stokes, ‘Electrolyte Solutions’, 2nd rev. edn., Butterworths, London (1965) pp. 155–60.
Ref. H. S. Harned and B. B. Owen, ‘The Physical Chemistry of Electrolytic Solutions’, 3rd end., Reinhold, New York (1958) pp. 510, 702.
T. Mussini,Chim. Ind. (Milan) 55 (1973) 637.
Ref. R. A. Robinson and R. H. Stokes, ‘Electrolyte Solutions’, 2nd rev. edn., Butterworths, London (1965), pp. 463–6.
D. E. Goldsack, R. Franchetto and A. Anttila Franchetto,Can. J. Amer. 54 (1976) 2953.
SAS User's Guide: Statistics, Version 5 edition. SAS Institute Inc., Cary N. C. (1985) pp 575, 655.
T. Mussini, C. Massarani-Formaro and P. Andrigo,J. Electroanal. Interf. Electrochem. 33 (1971) 189.
A. M. Azzam, PhD. Thesis, University of London, No. 441, (1949);Z. Elektrochem. 58 (1954) 889.
R. Cavaliere, P. Longhi, T. Mussini and S. Neglia,Gazz. Chim. Ital. 109 (1979) 399.
R. Cavaliere, P. Longhi, T. Mussini and S. Neglia,Gazz. Chim. Ital. 109 (1979) 495.
D. A. MacInnes, ‘The Principles of Eletrochemistry’, Reinhold, New York (1961) pp. 91–5.
Ref. R. A. Robinson and R. H. Stokes, ‘Electrolyte Solutions’, 2nd rev edn., Butterworths, London (1965) pp. 481, 491, 501, 504.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mussini, P.R., Longhi, P., Mussini, T. et al. Ion and solvent transfers at homoionic junctions between concentrated electrolyte solutions. J Appl Electrochem 20, 645–650 (1990). https://doi.org/10.1007/BF01008877
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01008877