Abstract
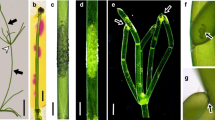
High-molecular-weight fluorochromes were intracellularly injected into a sieve element of the fascicular stem phloem ofVicia faba L., using a modified membrane-potential-recording pressure probe. After stabilization of the membrane potential following microelectrode impalement, either LYCH (Lucifer Yellow CH), 4.4-kDa FITC-dextran (fluoresceinisothiocyanate-dextran) conjugate, or 3-kDa, 10-kDa or 40-kDa LYCH-dextran conjugate was microinjected into the sieve element. Longitudinal fluorochrome movement across the sieve plates and lateral displacement to the companion cells was detected with all the probes except the 40-kDa conjugate. This indicates that the molecular exclusion limit of the pore/plasmodesma units between a sieve element and a companion cell in the fascicular stem phloem ofVicia faba lies between 10 kDa and 40 kDa.
Similar content being viewed by others
Abbreviations
- FITC:
-
fluoresceinisothiocyanate
- LYCH:
-
Lucifer Yellow CH
- MEL:
-
molecular exclusion limit
- PPU:
-
pore/plasmodesma unit
- SE/CC-complex:
-
sieve element/companion cell complex
References
Alosi MC, Melroy DL, Park RB (1988) The regulation of gelation of phloem exudate fromCucurbita fruit by dilution, glutathione, and glutathione reductase. Plant Physiol 86: 1089–1094
Barclay GF (1982) Slime plugs do not inhibit surge flow in sieve tubes. Can J Bot 60: 1281–1284
Böckenhoff A (1995) Untersuchungen zur Physiologie der Nährstoffversorgung des RübenzystennematodenHeterodera schachtii and von ihm induzierten Nährzellen in Wurzeln vonArabidopsis thaliana unter Verwendung einer speziell adaptierten ‘in situ’ Mikroinjektionstechnik. Ph.D. Thesis, University of Kiel, Germany
Bostwick DE, Dannenhoffer JM, Skaggs MI, Lister RM, Larkins BA, Thompson GA (1992) Pumpkin phloem lectin genes are specifically expressed in companion cells. Plant Cell 4: 1539–1548
Brault V, van den Heuvel JFJM, Verbeek M, Ziegler-Graff V, Reutenauer A, Herrbach E, Garaud J-C, Guilley H, Richards K, Jonard G (1995) Aphid transmission of beet western yellows luteovirus requires the minor capsid read-through protein P 74. EMBO J 14: 650–659
Citovsky V (1993) Probing plasmodesmatal transport with plant viruses. Plant Physiol 102: 1071–1076
Ding B, Haudenshield JS, Willmitzer L, Lucas WJ (1993) Correlation between arrested secondary plasmodesmal development and onset of accelerated leaf senescence in yeast acid invertase transgenic tobacco plants. Plant J 4: 179–189
Epel BL (1994) Plasmodesmata: composition, structure and trafficking. Plant Mol Biol 26: 1343–1356
Esau K (1978) Developmental features of the primary phloem inPhaseolus vulgaris. Ann Bot 42: 1–13
Esau K, Thorsch J (1985) Sieve plate pores and plasmodesmata, the communication channels of the symplast: ultrastructural aspects and developmental relations. Am J Bot 72: 1641–1653
Evert RF (1990) Dicotyledons. In: Behnke H-D, Sjölund RD (eds) Sieve elements. Comparative structure, induction and development. Springer, Berlin, pp 103–137
Fisher DB (1975) Structure of functional soybean sieve elements. Plant Physiol 56: 555–569
Fisher DB, Wu Y, Ku MSB (1992) Turnover of soluble proteins in the wheat sieve tube. Plant Physiol 100: 1433–1441
Goodwin PB (1983) Molecular size exclusion limit for movement in the symplast of theElodea leaf. Planta 157: 124–130
Hayes PM, Offler CE, Patrick JW (1985) Cellular structures, plasma membrane surface areas and plasmodesmatal frequencies of the stem ofPhaseolus vulgaris L. in relation to radial photosynthate transfer. Ann Bot 56: 125–138
Hull R (1989) The movement of viruses in plants. Annu Rev Phytopathol 24: 213–240
Jensen SG (1969) Occurrence of virus particles in the phloem tissue of BYDV-infected barley. Virology 38: 83–88
Kempers R, Prior DAM, Van Bel AJE, Oparka KJ (1993) Plasmodesmata between sieve element and companion cell of extrafascicular stem phloem ofCucurbita maxima permit passage of 3 kDa fluorescent probes. Plant J 4: 567–575
Lawton DM (1978) Ultrastructural comparison of the tailed and tailless P-protein crystals respectively of runner bean (Phaseolus multiflorus) and garden pea (Pisum sativum) with tilting stage electron microscopy. Protoplasma 97: 1–11
Lehmann J (1979) Nachweis von ATP und ATP-ase in den Siebröhren vonCucurbita pepo. Z Pflanzenphysiol 94: 331–338
Lucas WJ, Ding B, Van der Schoot C (1993) Plasmodesmata and the supracellular nature of plants. New Phytol 125: 435–476
Martin RR, Keese PK, Young MJ, Waterhouse PM, Gerlach WL (1990) Evolution and molecular biology of luteoviruses. Annu Rev Phytopathol 28: 341–363
Metcalfe CR, Chalk L (1988) Anatomy of dicotyledons. In: Metcalfe CR, Chalk L (eds) Systematic anatomy of leaf and stem, vol 1, 2nd edn. Claredon Press, Oxford
Miller WA (1994) Luteoviruses. In: Webster RG, Granoff A (eds) Encyclopedia of virology. Academic Press, London, pp 792–798
Northcote DH (1995) Aspects of vascular tissue differentiation in plants: parameters that may be used to monitor the process. Int J Plant Sci 156: 245–256
Oparka KJ (1991) Uptake and compartmentation of fluorescent probes by plant cells. J Exp Bot 42: 565–579
Oparka KJ, Murphy R, Derrick PM, Prior DAM, Smith JAC (1991) Modification of the pressure-probe technique permits controlled intracellular microinjection of fluorescent probes. J Cell Sci 98: 539–544
Oparka KJ, Viola R, Wright KM, Prior DAM (1992) Sugar transport and metabolism in the potato tuber. In: Pollock CJ, Farrar J, Gordon AJ (eds) Carbon partitioning within and between organisms. Bios, Oxford, pp 91–114
Oparka KJ, Duckett CM, Prior DAM, Fisher DB (1994) Real-time imaging of phloem unloading in the root tip ofArabidopsis. Plant J 6: 759–766
Patrick JW, Offler CE (1996) Post-sieve element transport of photoassimilates in sink regions. J Exp Bot 47 (Special issue): 1165–1177
Raven JA (1991) Long-term functioning of enucleate sieve elements: possible mechanisms of damage avoidance and damage repair. Plant Cell Env 14: 139–146
Resch A (1954) Beiträge zur Cytologic des Phloems, Entwicklungsgeschichte der Siebröhrenglieder und Geleitzellen betVicia faba L. Planta 44: 75–98
Rohde W, Gramstat A, Schmitz J, Tacke E, Prüfer D (1994) Plant viruses as model systems for the study of non-canonical translation mechanisms in higher plants. J Gen Virol 75: 2141–2149
Sakuth T, Schobert C, Pecsvaradi A, Eichholz A, Komor E, Orlich G (1993) Specific proteins in the sieve-tube exudate ofRicinus communis L. seedlings: separation, characterization and in-vivo labelling. Planta 191: 207–213
Schobert C, Großmann P, Gottschalk M, Komor E, Pecsvaradi A, zur Nieden U (1995) Sieve-tube exudate fromRicinus communis L. seedlings contains ubiquitin and chaperones. Planta 196: 205–210
Smith LM, Sabnis DD, Johnson RPC (1987) Immunocytochemical localisation of phloem lectin fromCucurbita maxima using peroxidase and colloidal-gold labels. Planta 170: 461–470
Tacke E, Prüfer D, Schmitz J, Rohde W (1991) The potato leafroll luteovirus 17 K protein is a single-stranded nucleic acid-binding protein. J Gen Virol 72: 2035–2038
Tacke E, Schmitz J, Prüfer D, Rohde W (1993) Mutational analysis of the nucleic acid-binding 17 kDa phosphoprotein of potato leafroll luteovirus identifies an amphipathic α-helix as the domain for protein/protein interactions. Virology 197: 274–282
Van Bel AJE (1993) The transport phloem. Specifics of its functioning. Progr Bot 54: 134–150
Van Bel AJE (1996) Interaction between sieve element and companion cell and the consequences for photoassimilate distribution. Two structural hardware frames with associated software packages in dicotyledons? J Exp Bot 47 (Special issue): 1129–1140
Van Bel AJE, Kempers R (1991) Symplastic isolation of the sieve element-companion cell complex in the phloem ofRicinus communis andSalix alba stems. Planta 183: 69–76
Van Bel AJE, Kempers R (1996) The pore/plasmodesm unit; key element in the interplay between sieve element and companion cell. Progr Bot 58, in press
Van Bel AJE, Van Rijen HVM (1994) Microelectrode-recorded development of the symplasmic autonomy of the sieve element/companion cell complex in the stem phloem ofLupinus luteus L. Planta 192: 165–175
Van der Schoot C, Van Bel AJE (1989) Glass microelectrode measurements of sieve tube membrane potentials in internodes and petioles of tomato (Solarium lycopersicum). Protoplasma 149: 144–154
Van der Schoot C, Van Bel AJE (1990) Mapping membrane potentials and dye coupling in internodal tissues of tomato (Solarium lycopersicum L.). Planta 182: 9–21
Van der Schoot C, Dietrich MA, Storms M, Verbeke JA, Lucas WJ (1995) Establishment of a cell-to-cell communication pathway between separate carpels during gynoecium development. Planta 195: 450–455
Wark MC, Chambers TC (1965) Fine structure of the phloem ofPisum sativum. I. The sieve element ontogeny. Aust J Bot 13: 171–183
Wooding FBP, Northcote DH (1965) The fine structure and development of the companion cell of the phloem ofAcer pseudoplatanus. J Cell Biol 24: 117–128
Zambryski P (1995) Plasmodesmata: plant channels for molecules on the move. Science 270: 1943–1944
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kempers, R., van Bel, A.J.E. Symplasmic connections between sieve element and companion cell in the stem phloem ofVicia faba L. have a molecular exclusion limit of at least 10 kDa. Planta 201, 195–201 (1997). https://doi.org/10.1007/BF01007704
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01007704