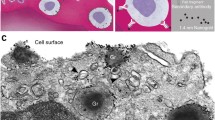
Summary
A triple ultrastructural immunogold staining method for the simultaneous demonstration of three surface antigens of peripheral blood mononuclear cells at the electron microscope level is described. A six-step pre-embedding immunoelectron microscopy procedure was developed, using commercially available reagents. The CD11b antigen was first detected, through a two-step (indirect) method with 40 nm-sized gold particles; after a blocking step, the HLA-DR surface antigen was subsequently detected, through a two-step (biotin-streptavidin) method with 20 nm-sized gold particles; the CD4 antigen was finally detected, through a one-step (direct) method, using 5 nm-sized gold particles. Electron microscopic examination revealed firstly the presence of a triple-labelled cell subpopulation, which showed gold granules of the three sizes simultaneously decorating the cell membrane. Thus, the cells of such a subset simultaneously expressed the three antigens investigated. In contrast, either gold particles of only one size or no gold particles were observed on the cell surface of other subpopulations. This technique is a model demonstrating the importance of varying the size of particles in pre-embedding gold immunoelectron microscopy for a better analysis of the expression of surface antigens in isolated cells.
Similar content being viewed by others
References
Bienz, K., Egger, D. &Pasamontes, L. (1986) Electron microscopic immunocytochemistry. Silver enhancement of colloidal gold marker allows double labeling with the same primary antibody.J. Histochem. Cytochem. 34, 1337–42.
Boyum, A. (1968) Isolation of mononuclear cells and granulocytes from human blood.Scand. J. Clin., Lab. Invest. 97, 77–89.
Chess, L., Evans, R., Humphreys, R. E., Strominger, J. L. &Schlossman, S. F. (1976) Inhibition of antibodydependent cellular cytotoxicity and immunoglobulin synthesis by an antiserum prepared against human B-cell Ia-like molecule.J. Exp. Med. 144, 113–22.
Courtoy, P. J., Picton, D. H. &Farquhar, M. G. (1983) Resolution and limitations of the immunoperoxidase procedure in the localization of extracellular matrix antigens.J. Histochem. Cytochem. 31, 945–51.
De Panfilis, G., Ferrari, C. &Manara, G. C. (1986a) Anin situ immunogold method applied to the identification of plasma membrane-associated antigens of skin-infiltrating cells.J. Invest. Dermatol. 87, 510–4.
De Panfilis, G., Manara, G. C. &Ferrari, C. (1986b) Immunogold labelling of epidermal Langerhans cells on tissue sections of normal human skin.Brit. J. Dermatol. 115, 351–6.
De Panfilis, G., Manara, G. C., Ferrari, C., Torresani, C. &Sansoni, P. (1988) Halry cell leukemia cells express CD1a antigen.Cancer 61, 52–7.
Dezutter-Dambuyant, C., Cordier, G., Schmitt, D., Faure, M., Laquoi, C. &Thivolet, J. (1984) Quantitative evaluation of two distinct cell populations expressing HLA-DR antigens in normal human epidermis.Brit. J. Dermatol. 111, 1–11.
Doerr-Schott, J. &Lichte, C. M. (1986) A triple ultrastructural immunogold staining method. Application to the simultaneous demonstration of three hypophyseal hormones.J. Histochem. Cytochem. 34, 1101–4.
Faulk, W. P. &Taylor, G. M. (1971) An immunocolloidal method for the electron microscope.Immunochemistry 8, 1081–3.
Ferrarini, M., Cadoni, A., Franzi, A. T., Ghiglioti, C., Leprini, A., Zicca, A. &Grossi, C. E. (1980) Ultrastructure and cytochemistry of human peripheral blood lymphocytes. Similarities between the cells of the third population and Tg lymphocytes.Eur. J. Immunol. 10, 562–70.
Grossi, C. E., Webb, S. R., Zicca, A., Lydyard, P. M., Moretta, L., Mingari, M. C. &Cooper, M. D. (1978) Morphological and histochemical analysis of two human T-cell populations bearing receptors for IgM or IgG.J. Exp. Med. 147, 1405–17.
Heitzmann, H. &Richards, F. M. (1974) Use of avidinbiotin complex for specific staining of biological membranes in electron microscopy.Proc. natn. Acad. Sci. USA. 71, 3537–41.
Horisberger, M. (1979) Evaluation of colloidal gold as a cytochemical marker for transmission and scanning electronmicroscopy.Biol. Cell. 36, 253–8.
Horisberger, M. &Rosset, J. (1977) Colloidal gold, a useful marker for transmission and scanning electron microscopy.J. Histochem. Cytochem. 25, 295–305.
Horisberger, M. &Vonlanthen, M. (1979) Multiple marking of cell surface receptors by gold granules: simultaneous localization of three lectin receptors on human erythrocytes.J. Microsc. 115, 97–102.
Karnovsky, M. J. (1971) Use of ferrocyanide reduced osmium tetroxide in electron microscopy.J. Cell. Biol. 51, 284.
Kohler, G. &Milstein, C. (1975). Continuous cultures of fused cells secreting antibody of predefined specificity.Nature 256, 495–7.
Landay, A., Gartland, G. L. &Clement, L. T. (1983) Characterization of a phenotypically distinct subpopulation of Leu-2 cells that suppress T cell proliferative responses.J. Immunol. 131, 2757–61.
Manara, G. C., De Panfilis, G. &Ferrari, C. (1985) Ultrastructural characterization of human large granular lymphocyte subsets defined by the expression of HNK-1 (Leu-7), Leu-11, or both HNK-1 and Leu-11 antigens.J. Histochem. Cytochem. 33, 1129–34.
Manara, G. C., De Panfilis, G., Ferrari, C. &Scandroglio, R. (1984) Immunoperoxidase-immunogold double labelling in immunoelectromicroscopy of large granular lymphocytes.J. Immunol. Methods 75, 189–92.
Manara, G. C., Sansoni, P., Ferrari, C. &De Panfilis, G. (1986) Natural killer cells expressing the Leu-11 antigen display phagocytic activity for 2-aminoethylisothiouronium bromide hydrobromide treated sheep red blood cells.Lab. Invest. 55, 412–8.
Mann, D. L., Abelson, S. H. &Amos, D. B. (1975) Detection of antigens for B-lymphoid cultured cell lines with human allo-antisera.J. Exp. Med. 142, 84–9.
Matutes, E. &Catovsky, D. (1982) The fine structure of normal lymphocyte subpopulations. A study with monoclonal antibodies and the immunogold technique.Clin. Exp. Immunol. 50, 416–25.
Nakane, P. K. (1968) Simultaneous localization of multiple tissue antigens using the peroxidase-labeled antibody method: a study on the pituitary glands of the rat.J. Histochem. Cytochem. 16, 557–60.
Novikoff, A. B. (1980) DAB cytochemistry: artifact problems in its current uses.J. Histochem. Cytochem. 28, 1036–7.
Picut, C. A., Lee, C. S., Dougherty, E. P., Andresen, K. L. &Lewis, R. M. (1987) Purification and identification of murine epidermal dendritic cells: flow cytometry and immunogold labeling.J. Histochem. Cytochem. 35, 745–53.
Reinherz, E. I., Kung, P. C., Goldstein, G. &Schlossman, S. F. (1979) Fuether characterization of the human inducer T cell subset defined by monoclonal antibody.J. Immunol. 123, 2894–6.
Romano, E. L. &Romano, M. (1977) Staphylococcal protein A bound to colloidal gold: a useful reagent to label antigen-antibody sites in electron microscopy.Immunochemistry 14, 711–15.
Roth, J., Bendayan, M. &Orci, L. (1978) Ultrastructural localization of intracellular antigens by the use of protein A-gold complex.J. Histochem. Cytochem. 26, 1074–81.
Roth, J. &Binder, M. (1978) Colloidal gold, ferritin and peroxidase as markers for electron microscopic double labeling lectin techniques.J. Histochem. Cytochem. 26, 169–9.
Roth, J. &Wagner, M. (1977) Redistribution and internalization of anti-AH1e and Concanavalin A-binding sites. An electron microscopic double labeling study.Exp. Pathol. 14, 311–20.
Schlossman, S. F., Chess, I., Humphreys, R. E. &Strominger, J. L. (1976) Distribution of Ia-like molecules on the surface of normal and leukemic cells.Proc. natn. Acad. Sci. USA 73, 1288–92.
Schmitt, D., Faure, M., Dambuyant-Dezutter, C. &Thivolet, J. (1984) The semi-quantitative distribution of T4 and T6 surface antigens on human Langerhans cells.Brit. J. Dermatol. 111, 655–61.
Staines, W. A., Meister, B., Melander, T., Nagy, J. I. &Hokfelt, T. (1988) Three-color immunofluorescence histochemistry allowing triple labeling within a single section.J. Histochem. Cytochem. 36, 145–51.
Timonen, T., Ortaldo, J. R. &Herberman, R. B. (1981) Characteristics of human large granular lymphocytes and relationship to natural killer and K cells.J. Exp. Med. 153, 569–82.
Van Der Loos, C., Das, P. K. &Houthoff, H. J. (1987) An immunoenzyme triple-staining method using both polyclonal and monoclonal antibodies from the same species. Applications of combined direct, indirect, and avidin-biotin complex (ABC) technique.J. Histochem. Cytochem. 35, 1199–204.
Wang, B. L. &Larsson, L. I. (1985) Simultaneous demonstration of multiple antigens by indirect immunofluorescence or immunogold staining. Novel light and electron microscopical double and triple staining method employing primary antibodies from the same species.Histochemistry 83, 47–56.
Winchester, R. J., Fu, S. M., Wernet, P., Kunkel, H. G., Dunpont, B. &Jersild, C. (1975) Recognition by pregnancy serums of non-HLA alloantigens selectively expressed on B lymphocytes.J. Exp. Med. 141, 924–9.
Wood, G. S., Warner, N. L. &Warnke, R. A. (1983) Anti-Leu 3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage.J. Immunol. 131, 212–6.
Zucker-Franklin, D. (1974) The percentage of monocytes among ‘mononuclear’ cell fractions obtained from normal human blood.J. Immunol. 112, 234–40.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
De Panfilis, G., Manara, G.C., Ferrari, C. et al. A multiple-staining ultrastructural procedure simultaneously detecting three membrane antigens on suspended cells by monoclonal antibodies and preembedding immunogold labelling. Histochem J 21, 136–144 (1989). https://doi.org/10.1007/BF01007488
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01007488