Summary
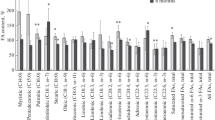
The effect of isoproterenol on myocardial metabolism in rats was studied using qualitative and quantitative histochemical techniques. The activity and location of 20 enzymes that play a role in the aerobic and anaerobic pathways of energy metabolism were qualitatively examined. The activity and location of some hydrolytic enzymes and the glycogen content were also qualitatively studied. For the quantitative study the activity of 10 enzymes was measured.
The isoproterenol injections induced necrosis with inflammatory infiltrates. The muscle fibres in the necrotic regions were characterized by defective aerobic energy metabolism and increased glycolytic capacity. There was a depletion of the glycogen reserves in the necrotic fibres. These fibres showed a markedly increased activity of enzymes belonging to the oxidative branch of the pentose phosphate pathway. The implication of this increase for the metabolism of the myocardial cells is discussed. The activity of acid phosphatase in the pathological muscle fibres was strongly increased. The inflammatory cells in the necrotic areas were characterized by preponderantly anaerobic metabolism.
Similar content being viewed by others
References
Abri, O. &Hecht, A. (1981) The influence of long-term application of isoproterenol on the results of temporary ischemia of the heart muscle.Exp. Pathol. 20, 146–52.
Autor, A. P. (1982)Pathology of Oxygen. London-New York: Academic Press.
Barka, M. D. &Anderson, P. J. (1963)Histochemistry, Theory, Practice and Bibliography. New York: Harper and Row.
Barner, H. B., Jellinek, M. &Kaiser, G. C. (1970) Effects of isoproterenol infusion of myocardial structure and composition.Am. Heart J. 79, 237–43.
Bloom, S. &Davis, D. (1974)Myocardial Biology, München, Berlin, Wien: Verlag Urban und Schwarzenberg.
Bodaness, R. S. (1982) The potential role of NADPH and cytoplasmic NADP-linked dehydrogenases in protection against singlet oxygen mediated cellular toxicity.Biochem. Biophys. Res. Commun. 108, 1709–15.
Bors, W., Michel, CL, Saran, M. &Lengfelder, E. (1978) The involvement of oxygen radicals during the autooxidation of adrenalin.Biochim. Biophys. Acta 540, 162–72.
Buckberg, G. D. &Ross, G. (1973) Effects of isoproterenol on coronary blood flow. Its distribution and myocardial performance.Cardiovasc.Res. 7, 429–37.
Burstone, M. S. (1962)Enzyme Histochemistry, New York: Academic Press.
Busch, H. F. M., Jennekens, F. G. I. &Scholte, H. R. (1981)Mitochondria and Muscular Diseases. Beetsterwaag, The Netherlands: Mefar B. V.
Chappel, C. I., Rona, G., Balasz, T. &Gaudry, R. (1959) Severe myocardial necrosis produced by isoproterenol in the rat.Arch. Int. Pharmacodyn. Ther. 122, 123–8.
Ciplea, A. G. &Bock, P. R. (1976a) Qualitative und quantitative histoenzymatische Studie and den durch Isoproterenol induzierten Myokardnekrosen bei Ratten.Arzneimittel-Forschung 26, 799–812.
Ciplea, A.G. &Bock, P. R. (1976b) Kardioprotektion durch perorale Anwendung des Calcium-Antagonisten Sensit. Verhütung isoproterenol-bedingter Myokardnekrosen bei Ratten.Arzneimittel-Forschung 26, 1819–26.
Ciplea, A. G., Metze, K., Grundmann, E., Roessner, A. &Hettwer, H. (1985) Microphotometric quantitation of enzyme activities in giant cell tumor of bone.Pathol Res. Pract. 179, 412–8.
Danielsson, H. &Tchen, T. T. (1968) Steroid metabolism. InMetabolic Pathways, Vol. 2. (edited byGreenberg, D. M.) pp. 116–98. London, New York: Academic Press.
Davies, M. J. (1977) The pathology of myocardial ischemia.J. Clin. Pathol. 30, (suppl. 11), 45–52.
De Vries, G. P. &Meijer, A. E. F. H. (1976) Semipermeable membranes for improving the histochemical demonstration of enzyme activities in tissue section. 6.D-glucose-6-phosphate isomerase and phosphogluco-mutase.Histochemistry 50, 1–8.
De Vries, G. P., Tigges, A. J. &Meijer, A. E. F. H. (1980) The histochemical demonstration of glyceraldehyde phosphate dehydrogenase activity with a semipermeable membrane technique.Histochem. J. 12, 119–22.
Drummond, G. I., Duncan, L. &Hertzman, E. (1966) Effect of epinephrine on phosphorylaseb kinase in perfused rat hearts.J. biol. Chem. 241, 5899–903.
Elias, E. A. (1985) Metabolic studies as a diagnostic measure for cancer. 1. Adenocarcinomas of different organs, especially the human mamma.Cell. Molec. Biol. 31, 281–98.
Elias, E. A., Elias, R. A., De Vries, G. P. &Meijer, A. E. F. H. (1982) Early and late changes in the metabolic pattern of the working myocardial fibres and Purkinje fibres of the human heart under ischaemic and inflammatory conditions; an enzyme histochemical study.Histochem. J. 14, 445–59.
Elias, E. A., Elias, R. A., Kooistra, A. M., Roukema, A. C., Blacquiere, J. F., Barrowclough, H. &Meijer, A. E. F. H. (1983) Fluctuations in the enzymatic activity of the human endometrium.Histochemistry 77, 159–70.
Elias, E. A. &Meijer, A. E. F. H. (1981) The increase in activity of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in skeletal muscles of rats after subcutaneous administration ofN, N 1-dimethy-para-phenylenediamine.Histochemistry 71, 543–58.
Ferrans, V. J., Hibbs, R. G., Black, W. C. &Weilbacher, D. G. (1964) Isoproterenol-induced myocardial necrosis A histochemical and electron microscopic study.Am. Heart J. 68, 71–90.
Fields, M. &Laufer, A. (1977) Lysosomal enzyme release in the isolated perfused heart of immunized rats.J. Molec. Cell. Cardiol. 9, 933–43.
Fleckenstein, A., Pachinger, O., Leder, O., Hein, B. &Janke, J. (1972) Verhütung Isoproterenol-induzierter Myokardnekrosen durch Senkung des Plasma Calcium Spiegels mittels Calcitonin.Pflügers Arch. Ges. Physiol. 332, (suppl.) R40.
Freeman, B. A. &Carpo, J. D. (1982) Biology of disease. Free redicals and tissue injury.Lab. Invest. 47, 412–26.
Glenner, G. G., Burtner, H. J. &Brown, G. W. (1957) The histochemical demonstration of monoamine oxidase activity by tetrazolium salts.J. Histochem. Cytochem. 5, 591–600.
Gopinath, C., Thuring, J. &Zayed, I. (1978) Isoprenaline-induced myocardial necrosis in dogs.Br. J. Exp. Pathol. 59, 148–57.
Green, D. E. &Allmann, D. W. (1968) Biosynthesis of fatty acids. InMetabolic Pathways, Vol. 2. (edited byGreenberg, D. M.) pp. 38–67. London, New York: Academic Press.
Hack, M. H. &Helmy, F. M. (1967) Some correlative lipid and histochemical studies of experimental myocardial infarction in the dog.Acta Histochem. 27, 291–302.
Hammermeister, K. E., Yunis, A. A. &Krebs, E. G. (1965) Studies on phosphorylase activation in the heart.J. biol. Chem. 240, 986–91.
Hearse, D. J., Garlick, P. B., Humphrey, S. T. &Shillingford, J. P. (1978) The effect of drugs on enzyme release from the hypoxic myocardium.Eur. J. Cardiol. 7, 421–36.
Hettwer, H. (1984) The Ca2+-activated ATP-A in the tubuli seminiferi of rats.Cell. Molec. Biol. 30, 391–9.
Horecker, B. L. (1968) Pentose phosphate pathway, uronic acid pathway, interconversion of sugars. InCarbohydrate Metabolism, Vol. 1 (edited byDickens, F., Whelan, W. J. &Randle, P. J.) pp. 137–67. London, New York: Academic Press.
Jodalen, H., Lie, R. &Rotevatn, S. (1982) Effect of isoproterenol on lipid accumulation in myocardial cells.Res. Exp. Med. (Berlin) 181, 239–44.
Judd, J. T. &Wexler, B. C. (1969) Myocardial connective tissue metabolism in response to injury: histological and chemical studies of mucopolysaccharide and collagen in rat hearts after isoproterenol induced infarction.Circ. Res. 25, 201–14.
Kahn, D. S., Rona, G. &Chappel, C. I. (1969) Isoproterenol-induced cardiac necrosis.Ann. N.Y. Acad. Sci. 156, 285–93.
Kapuscinski, M. &Williams, J. F. (1981) There is an active and extensive pentose phosphate pathway of glucose metabolism in rat hearts.J. Molec. Cell. Cardiol. 13, 4.
Kief, H. &Bähr, H. (1975) Zur experimentellen Pathologie der Ischämie an Herzen.J. Angiologisches Symposium. pp. 31–9, Stuttgart, New York: Schattauer-Verlag.
Krause, E.-G. &England, P. J. (1982) Loss of the cyclic AMP accumulation induced by isoproterenol ischaemie in the isolated rat heart.J. Molec. Cell. Cardiol. 14, 611–3.
Krause, E.-G. &England, P. J. (1984) Effect of phosphorylation on protein phosphorylation in myocardial ischaemia.Gen. Physiol. Biophys. 3, 193–9.
Lange, P. W. &Engström, A. (1954) Determination of thickness of microscopic objects.Lab. Invest. 3, 116–31.
Lojda, Z., Gossrau, R. &Schiebler, T. H. (1979)Enzyme Histochemistry Berlin, Heidelberg, New York: Springer Verlag.
Loschen, G. &Flohe, L. (1971) Respiratory chain linked H2O2 production in pigeon heart mitochondria.FEBS Lett. 18, 261–4.
Meerson, F. Z., Kagan, V. E., Kozlov, Y. P., Belkina, L. M. &Arkhipenko, Y. V. (1982) The role of lipid peroxidation in pathogenesis of ischemic damage and the antioxidant protection of the heart.Basic Rec. Cardiol. 77, 465–85.
Meijer, A. E. F. H. (1970) Histochemical method for the demonstration of myosin adenosine triphosphatase in muscle tissue.Histochemie 22, 51–8.
Meijer, A. E. F. H. (1972) Semipermeable membranes for improving the histochemical demonstration of enzyme activities in tissue sections. I. Acid phosphatase.Histochemie 30, 31–9.
Meijer, A. E. F. H. (1973) Semipermeable membranes for improving the histochemical demonstration of enzyme activities in tissue sections. III. Lactate dehydrogenase.Histochemie 35, 165–72.
Meijer, A. E. F. H. (1980) Semipermeable membrane techniques in quantitative enzyme histochemistry. InTrends in Enzyme Histochemistry and Cytochemistry. Ciba Foundation Symposium73, pp. 103–20. Amsterdam: Excerpta Medica.
Meijer, A. E. F. H., Benson, D. &Scholte, H. R. (1977b) The influence of freezing and freeze-drying of tissue specimens on enzyme activity.Histochemistry 51, 297–303.
Meijer, A. E. F. H. &De Vries, G. P. (1974) Semipermeable membranes for improving the histochemical demonstration of enzyme activities in tissue sections. IV. Glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase (decarboxylating).Histochemistry 40, 349–59.
Meijer, A. E. F. H. &De Vries, G. P. (1975) Semipermeable membranes for improving the histochemical demonstration of enzyme activities in tissue sections. V. Isocitrate: NADP+ oxidoreductase (decarboxylating) and malate:NADP+ oxidoreductase (decarboxylating).Histochemistry 43, 225–36.
Meijer, A. E. F. H. &Elias, E. A. (1977) Die Aktivität der Glucose-6-phosphat-Dehydrogenase und 6-phosphogluconat-Dehydrogenase in Skelettmuskelgewebe von Patienten mit Muskelkrankheiten.Acta Histochem. Suppl.18, 169–75.
Meijer, A. E. F. H. &Elias, E. A. (1984a) The inhibitory effect of actinomycin D and cycloheximide on the increase in activity of glucose-6-phosphate dehydrogenase in experimentally induced diseased skeletal muscles.Histochem. J. 16, 971–82.
Meijer, A. E. F. H. &Elias, E. A. (1984b) Die Bedeutung der Kapazitätszunahme des Pentosephosphatzyklus in malignen Tumoren für den Energiestoffwechsel.Acta Histochem. Suppl.29, 141–8.
Meijer, A. E. F. H. &Elias, E. A. (1985) Kapazitätszunahme des Pentosephosphatzyklus in pathologisch veränderten Skelettmuskelfasern.Wiss. Z. Friedrich-Schiller-Universität Jena 34, 396–405.
Meijer, A. E. F. H., Elias, E. A. &Vloedman, A. H. T. (1977a) The value of enzyme histochemical techniques in classifying fibre types of human skeletal muscle. 3. Human skeletal muscles with inherited or acquired disease of the neuromuscular system.Histochemie 53, 97–105.
Meijer, A. E. F. H. &Stegehuis, F. (1980) Histochemical technique for the demonstration of phosphofructokinase activity in heart and skeletal muscles.Histochemistry 66, 75–81.
Meijer, A. E. F. H. &Vloedman, A. H. T. (1973) Semipermeable membranes for improving the histochemical demonstration of enzyme activities in tissue sections. II. Non-specific esterase and β-glucuronidase.Histochemie 34, 127–34.
Meijer, A. E. F. H. &Vloedman, A. H. T. (1980) The histochemical characterization of the coupling state of skeletal muscle mitochondria.Histochemistry 69, 217–32.
Mistra, H. P. &Fridovich, I. (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase.J. biol. Chem. 247, 3170–5.
Nachlas, M., Tsou, K. C., De Souza, E., Cheng, C.-S. &Seligman, A. M. (1957) The cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted dinitrazole.J. Histochem. Cytochem. 5, 420–36.
Nohl, H. &Hegner, D. (1978) Do mitochondria produce oxygen radicalsin vivo? Eur. J. Biochem. 82, 563–7.
Rona, G., Chappel, C. I., Balasz, T. &Gaudry, R. (1959) An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol on the rats.Arch. Pathol. 67, 443–55.
Rona, G., Chappel, C. I. &Kahn, D. S. (1963) The significance of factors modifying the development of isoproterenol-induced myocardial necrosis.Am. Heart J. 66, 389–95.
Rowe, T. G., Manson, N. H., Caplan, M. &Hess, M. L. (1983) Hydrogen peroxide and hydroxyl radical medication of activated leucocyte depression of cardial sarcoplasmic reticulum.Circ. Res. 33, 584–91.
Saroff, J. &Wexler, B. C. (1970) Isoproterenol-induced myocardial infarction in rats: distribution of corticosterone.Circ. Res. 27, 1101–9.
Seidler, E. (1983) Zum histochemischen Nachweis von Gewebsalterationen durch Tetrazoliumsalze unterschieldlicher Nitrosencitivität.Acta Histochem. 73, 219–25.
Shnitka, T. &Nachlas, M. M. (1963) Histochemical alteration in ischemic heart muscle and early myocardial infarction.Am. J. Pathol. 5, 507–25.
Sharma, R. V., Gupta, R. C., Ramanadham, M., Venema, R. C. &Bhalla, C. (1983) Reduced cAMP levels and glycogen phosphorylase activation in isoproterenol perfused SHR myocardium.Basic Res. Cardiol. 78, 695–705.
Shlaver, M., Kane, P. F., Wiggins, V. Y. &Kirsh, M. M. (1982) Possible role for cytotoxic oxygen metabolites in the pathogenesis of cardiac ischaemic injury.Circulation 66, 185–92.
Stanton, H. C., Brenner, G. &Mayfield, E. D. (1969) Studies of isoproterenol-induced cardiomegaly in rats.Am. Heart J. 77, 72–80.
Stewart, J. R., Blackwell, W. H., Crute, S. L., Loughlin, V., Hess, M. L. &Greenfield, L. J. (1982) Prevention of myocardial ischemia-reperfusion injury with oxygen free radical scavengers.Surg. Forum. 33, 317–21.
Stull, J. T. (1980) Phosphorylation of contractive proteins in relation to muscle function.Adv. Cycl. Nucleotide Res. 13, 39–94.
Tajuddin, M., Ahmad, M. &Tariq, M. (1975) Effect of conditioning on myocardial metabolism after myocardial infarction. InRecent Advances in Studies on Cardiac Structure and Metabolism, Vol. 10 pp. 561–68. Baltimore: University Park Press.
Titus, E. O. (1983) A molecular biologic approach to cardiac toxicology.Adv. Exp. Med. Biol. 161, 509–18.
Todd, G. L., Pieper, G. M., Clayton, F. C. &Eliot, R. S. (1979) Heterogeneity in distribution of cardiac glycogen following isoproterenol infusions in the dog.Histochem. J. 11, 425–34.
Van Den Hoven, R., Wensing, Th., Breukink, H. J. &Meijer, A. E. F. H. (1987) Enzyme histochemistry in exertional rhabdomyolysis. InEquine Exercise Physiology (edited byGillespie, J. R. &Robinson, N. E.) pp. 796–810. Davis, California: ICEEP publications.
Wexler, B. C. (1978) Myocardial infarction in young and old male rats; pathophysiological changes.Am. Heart J. 96, 70–80.
Wildenthal, K. (1975) Lysosomes and lysosomal enzymes in the heart. InLysosomes in Biology and Pathology, Vol. 4. (edited byDingle J. T. &Dean, R. T.) pp. 167–70. Amsterdam, Oxford: North Holland.
Williams, B. J. &Mayer, S. E. (1966) Hormonal effects on glycogen metabolism in the rat heartin situ.Molec. Pharmacol. 2, 454–64.
Wood, T. (1985)The Pentose Phosphate Pathway, Orlando: Academic Press.
Wood, W. G., Lindenmayer, G. E. &Schwartz, A. (1971) Myocardial synthesis of ribonucleic acid. 1. Stimulation by isoproterenol.J. Molec. Cell. Cardiol. 3, 127–38.
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. H. G. Goslar in honour of his 70th birthday.
Rights and permissions
About this article
Cite this article
Meijer, A.E.F.H., Hettwer, H. & Ciplea, A.G. An enzyme histochemical study of isoproterenol-induced myocardial necroses in rats. Histochem J 20, 697–707 (1988). https://doi.org/10.1007/BF01002750
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01002750