Abstract
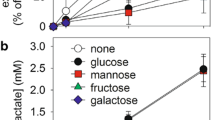
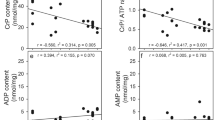
Exposure of rat pheochromocytoma PC12 cells to 0.1 mM 6-aminonicotinamide (6AN) for 24 hours resulted in a 500-fold increase in 6-phosphogluconate indicating active metabolism of glucose via the oxidative enzymes of the pentose phosphate pathway. Amounts of 6-phosphogluconate that accumulated in 6AN-treated cells at 24 hours were significantly increased by treatment of the cells with nerve growth factor (NGF) (100 ng 7S/ml) suggesting that metabolism of glucose via the pentose pathway at this time was enhanced by NGF. This stimulation of metabolism via the pentose pathway is probably a late response to NGF because initial rates of 6-phosphogluconate accumulation in 6AN-treated cells were the same in the presence and absence of NGF. Moreover, amounts of14CO2 generated from 1-[14CO2]glucose during the initial six hour incubation period were the same in control and NGF-treated cells. Specific activities of hexose phosphates labeled from 1-[14CO2]glucose were also the same in control and NGF-treated cells. The observation that 6AN inhibited metabolism via the pentose phosphate pathway but failed to inhibit NGF-stimulated neurite outgrowth suggests that NADPH required for lipid biosynthesis accompanying NGF-stimulated neurite outgrowth from PC12 cells can be derived from sources other than, or in addition to, the oxidative enzymes of the pentose phosphate pathway.
Similar content being viewed by others
References
Cohen, S. 1959. Purification and metabolic effects of a nerve growth-promoting protein from snake venom. J. Biol. Chem. 234:1129–1137.
Angeletti, P. U., Luizzi, A., Levi-Montalcini, R., and Gandini-Attardi, D. 1964. Effect of a nerve growth factor on glucose metabolism by sympathetic and sensory nerve cells. Biochim. Biophys. Acta 90:445–450.
Larrabee, M. G. 1972. Metabolism during development in sympathetic ganglia of chickens: Effects of age, nerve growth factor and metabolic inhibitors. Pages 70–88. in Zaimis, E. and Knight, J. (eds.), Nerve growth factor and its antiserum, Athlone Press, London.
Köhler, E., Barrach, H-J., and Neubert, D. 1970. Inhibition of NADP dependent oxidoreductases by the 6-aminonicotinamide analogue of NADP. FEBS Lett. 6:225–228.
Lange, K., Kolbe, H., Keller, K., and Herken, H. 1970. Der Kohlenhydratstoffwechsel des Gehirns nach Blockade des Pentose-PhosphatWeges durch 6-Aminonicotinamid. Hoppe-Seyler's Z. Physiol. Chem. 351:1241–1252.
Kauffman, F. C., and Johnson, E. C. 1974. Cerebral energy reserves and glycolysis in neural tissue of 6-aminonicotinamide-treated mice. J. Neurobiol. 5:379–392.
Härkönen, M. H. A., and Kauffman, F. C. 1974. Metabolic alterations in the axotomized superior cervical ganglion of the rat. II. Pentose phosphate pathway. Brain Res. 65:141–157.
Guroff, G. 1985. PC12 cells as a model of neuronal differentiation. in Bottenstein, J., and Sato, G. (eds.), Cell culture in the neurosciences, Plenum Press, New York.
Greene, L. A., and Tischler, A. S. 1977. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 73:2424–2428.
Lowry, O. H., and Passonneau, J. V. 1972. A flexible system of enzymatic analysis. Academic Press, New York.
Kauffman, F. C., Brown, J. G., Passonneau, J. V., and Lowry, O. H. 1969. Effects of changes in brain metabolism on levels of pentose phosphate pathway intermediates. J. Biol. Chem. 244:3647–3653.
Sinicropi, D. V., Dombrowski, A., Montgomery, C. W., Evans, R. K., and Kauffman, F. C. 1980. Maintenance of the adult rat superior cervical ganglion in vitro: Comparison of organ and explant culture systems. J. Neurochem. 34:1280–1287.
Lowry, O. H., Roberts, N. R., Wu, M-L, Hixon, W. S., and Crawford, E. J. 1954. The quantitative histochemistry of brain. II. Enzyme measurements. J. Biol. Chem. 207:19–37.
Tildon, J. T., Merrill, S., and Roeder, L. M. 1983. Differential substrate oxidation by dissociated brain cells and homogenates during development. Biochem. J. 216:21–25.
Kahana, S. E., Lowry, O. H., Schulz, D. W., Passonneau, J. V., and Crawford, E. J. 1960. The kinetics of phosphoglucoisomerase. J. Biol. Chem. 235:2178–2184.
Hostetler, K. Y., Landau, B. R., White, R. J., Albin, M. S., and Yashon, D. 1970. Contribution of the pentose cycle to the metabolism of glucose in the isolated perfused brain of the monkey. J. Neurochem. 17:33–39.
Sokal, R. R., and Rohlf, F. J. 1969. Biometry. Pages 1420–1457. W. H. Freeman and Co., San Francisco.
Author information
Authors and Affiliations
Additional information
Special Issue dedicated to Dr. O. H. Lowry.
Rights and permissions
About this article
Cite this article
Davis, L.H., Kauffman, F.C. Metabolism via the pentose phosphate pathway in rat pheochromocytoma PC12 cells: Effects of nerve growth factor and 6-aminonicotinamide. Neurochem Res 12, 521–527 (1987). https://doi.org/10.1007/BF01000236
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01000236