Conclusions
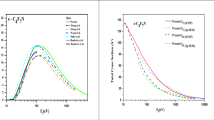
The values of D 00 obtained for sodium and potassium by the two methods are in good agreement. The value of D 00 for Na2 molecules agrees within tolerances with other measurement results, with the exception of the value obtained by the luminescent irradiation method, which is known to be rough. The value of D 00 obtained by us for a K2 molecule is in good agreement with the dissociation energy value obtained by the molecular beam method [12]. Spectroscopic measurements provide a much smaller value for D 00 , but according to Gaydon [13] they are not reliable, since they use too large an extrapolation of Berge-Sponer.
A slightly better agreement between the pressures calculated from (5) and the experimental values of sodium and potassium pressures are obtained in the values of D 00 and ΔH 010 calculated by the second method are used. Therefore, we can recommend the values of D 00 =17,920 ±400 kj/kmole (for an Na2 molecule), of D 00 =53,750±400 kj/kmole (for a K2 molecule), and corresponding heat of sublimation values of ΔH 010 =107,708 ±7 kj/kmole for sodium and of ΔH 010 =90,094 ±4 kj/kmole for potassium.
The tolerance of ±400 kj/kmole has been adopted by us so as to make the recommended value of D 00 agree with the results obtained by the molecular beam method for Na2 and K2, and by the spectrometric method for Na2.
Similar content being viewed by others
Literature cited
M. Sitting, Sodium, Its Production, Properties, and Application [Russian translation], Atomizdat, Moscow (1961).
M. Makansy, W. Selke, and C. Bonilla, J. Chem. Eng. Data,5, No. 4 (1960).
L. D. Volyak, IFZh,5, No. 7 (1962).
R. Thorn and G. Winslow, J. Phys. Chem.,65 (1961).
W. Evans, R. Jacobson, R. Munson, and R. Wagman, J. Res. NBS,55, No. 2 (1955).
É. É. Shpil'rain, Teplofiz. vus. temp., No. 2 (1966).
Yu. K. Vinogradov and L. D. Volyak, Teplofiz. vus. temp., No. 1 (1966).
V. P. Glushko, Editor, Thermodynamic Properties of Individual Substances [in Russian], Izd. AN SSSR, Moscow, (1962).
M. Makansy, C. Muendel, and W. Selke, J. Phys. Chem.,59 (1955).
J. Walling, J Phys. Chem.,67 (1963).
M. Makansy, M. Madsen, W. Selke, and C. Bonilla, J Phys. Chem.,60 (1956).
L. Lewis, Z. Phys.,69 (1931).
A. Gaydon, Dissociation Energies and Spectra of Diatomic Molecules, London (1953).
Additional information
Translated from Izmeritel'naya Tekhnika, No. 3, pp. 43–46, March, 1967.
Rights and permissions
About this article
Cite this article
Vinogradov, Y.K., Volyak, L.D. Evaluation of the dissociation energy of Na2 and K2 molecules from the saturated vapor pressure of sodium and potassium. Meas Tech 10, 318–321 (1967). https://doi.org/10.1007/BF00998300
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00998300