Conclusions
-
1.
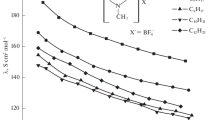
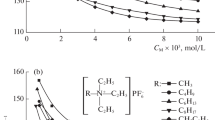
The ionic association of salts of trinitromethane, dinitromethane, and gem-dinitroethane was studied in solution of methanol and ethanol by the method of electric conductivity. The dissociation constants of the ion pairs of the salts were calculated. It was found that these values are of the same order of magnitude in methanol and in ethanol.
-
2.
The degree of association of the salts studied in methanol and ethanol, as a rule, does not depend on the nature of the cation and anion, which is evidence of the equalizing effect of these solvents.
-
3.
On the basis of the data obtained on the electric conductivity we compared the solvating ability of methanol and ethanol with respect to the anions studied: trinitromethane, dinitroethane.
-
4.
The interionic distances in the ion pairs were estimated.
Similar content being viewed by others
Literature cited
V. I. Slovetskii, A. F. Toshcheva, A. A. Fainzil'berg, S. A. Shevelev, and V. I. Erashko, Izv. Akad. Nauk SSSR, Ser. Khim., 1054 (1968).
A. F. Toshcheva, V. I. Slovetskii, A. A. Fainzil'berg, S. A. Shevelev, and V. I. Erashko, Izv. Akad. Nauk SSSR, Ser. Khim., 2484 (1968).
A. F. Baskakova, V. I. Slovetskii, S. A. Shevelev, V. I. Erashko, and A. A. Fainzil'berg, Izv. Akad. Nauk SSSR, Ser. Khim., 2404 (1969).
Yu. K. Yur'ev, Practical Studies in Organic Chemistry [in Russian], Izd-vo Mosk. Gos. Un-ta (1961), pp. 53, 57.
R. Robinson and R. Stokes, Solutions of Electrolytes [Russian translation], IL, Moscow (1963).
A. P. Kreshkov, L. N. Bykova, and M. A. Kazaryan, Titration of Inorganic and Organic Compounds in Nonaqueous Solutions [in Russian], Khimiya (1967), p. 43.
A. I. Ivanov, V. I. Slovetskii, S. A. Shevelev, A. A. Fainzil'berg, and S. S. Novikov, Zh. Fiz. Khimii, 830 (1967).
The Chemist's Handbook [in Russian], Khimiya (1964), p. 3.
R. Kay and D. Evans, J. Phys. Chem.,70, 2325 (1966).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 8, pp. 1768–1772, July, 1970.
Rights and permissions
About this article
Cite this article
Slovetskii, V.I., Baskakova, A.F., Shevelev, S.A. et al. Study of the ionic association of salts of nitro-compounds in solution. Russ Chem Bull 19, 1667–1670 (1970). https://doi.org/10.1007/BF00996501
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00996501