Abstract
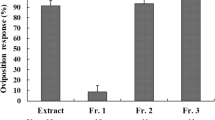
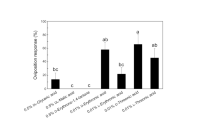
Oviposition by females of the pipevine swallowtail butterfly,Battus philenor, was stimulated by contact with alcoholic extracts of host foliage.d-(+)-Pinitol was isolated and identified from leaf material of one host species,Aristolochia macrophylla (Aristolochiaceae). In combination with chloroform-soluble components of host leaf material, this compound was comparable to the parent extract in stimulating oviposition.
Similar content being viewed by others
References
Bailey, L.H. 1949. Manual of Cultivated Plants, revised edition, Macmillan, New York.
Berridge, M.J., andIrvine, R.F. 1989. Inositol phosphates and cell signalling.Nature 341:197–205.
Binder, R.G., andHaddon, W.F. 1984. Analysis ofO-methylinositols by gas-liquid chromatog-raphy-mass spectrometry.Carbohyd. Res. 129:21–34.
Bush, G.L. 1975. Sympatric speciation in phytophagous parasitic insects, pp. 187–206,in P.W. Price (ed.). Evolutionary Strategies of Parasitic Insects and Mites. Plenum, New York.
Campbell, B.C., andBinder, R.G. 1984. Alfalfa cyclitols in the honeydew of an aphid.Phytochemistry 23:1786–1787.
Chen, Z., andZhu, D. 1987.Aristolochia alkaloids, pp. 29–65,in A. Brossi (ed.). The Alkaloids: Chemistry and Pharmacology, Vol. 31. Academic Press, New York.
David, W.A.L., andGardiner, B.O.C. 1962. Oviposition and the hatching of the eggs ofPieris brassicae (L.) in a laboratory culture.Bull. Entomol. Res. 53:91–109.
Dethier, V.G. 1941. Chemical factors determining the choice of food plants byPapilio larvae.Am. Nat. 75:617/83.
Dittrich, P., andBrandl, A. 1987. Revision of the pathway ofd-pinitol formation in Leguminosae.Phytochemistry 26:1925–1926.
Dittrich, P., andKorak, A. 1984. Novel biosynthesis ofd-pinitol inSimmondsia chinensis.Phytochemistry 23:65–66.
Dreyer, D.L., Binder, R.G., Chan, B.G., Weiss, A.C., Jr., Hartwig, E.E., andBeland, G.L. 1979. Pinitol, a larval growth inhibitor forHeliothis zea in soybeans.Experientia 35:1182–1183.
Ehrlich, P.R., andRaven, P.H. 1964. Butterflies and plants: a study in coevolution.Evolution 18:586–608.
Feeny, P.P. 1987. The roles of plant chemistry in associations between swallowtail butterflies and their host plants, pp. 353–359,in V. Labeyrie, G. Fabres and D. Lachaise, (eds.). InsectsPlants. W. Junk Publishers, Dordrecht, The Netherlands.
Feeny, P. 1991. Chemical constraints on the evolution of swallowtail butterflies, pp. 315–340,in P.W. Price, T.M. Lewinsohn, G.W. Fernandes and W.W. Benson (eds.). Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. John Wiley & Sons, New York.
Feeny, P., Rosenberry, L., andCarter, M. 1983. Chemical aspects of oviposition behavior in butterflies, pp. 27–76,in S. Ahmad (ed.). Herbivorous Insects: Host-seeking Behavior and Mechanisms. Academic Press, New York.
Feeny, P., Sachdev, K., Rosenberry, L., andCarter, M. 1988. Luteolin-7-O-(6″-O-malonyl)-β-d-glucoside and trans-chlorogenic acid: Oviposition stimulants for the black swallowtail butterfly.Phytochemistry 27:3439–3448.
Fraenkel, G.S. 1959. The raison d'être of secondary plant substances.Science 129:1466–1470.
Gardner, W.A., Phillips, D.V., andSmith, A.E. 1984. Effect of pinitol on the growth ofHeliothis zea andTrichoplusia ni larvae.J: Agric. Entomol. 1:101–105.
Hamamura, Y., Hayashiya, K., Naito, K., Matsura, K., andNishida, J. 1962. Food selection by silkworm larvae.Nature 194:754–755.
Honda, K. 1986. Flavanone glycosides as oviposition stimulants in a papilionid butterfly, Papilio protenor.J. Chem. Ecol. 12:1999–2010.
Honda, K. 1990. Identification of host-plant chemicals stimulating oviposition by swallowtail butterfly, Papilio protenor.J. Chem. Ecol. 16:325–337.
Ichinosé, T., andHonda, H. 1978. Ovipositional behavior ofPapilio protenor demetrius Cramer and the factors involved in its host plants.Appl. Entomol. Zool. 13:103–114.
Jermy, T. 1976. Insect-host-plant relationship—coevolution or sequential evolution? pp. 109–113,in T. Jermy (ed.). The Host-Plant in Relation to Insect Behaviour and Reproduction. Plenum, New York.
Jermy, T. 1984. Evolution of insect/host plant relationships.Am. Nat. 124:609–630.
Khattar, P., andSaxena, K.N. 1978. Interaction of visual and olfactory stimuli determining orientation ofPapilio demoleus larvae.J. Insect Physiol. 24:571–576.
Kindl, H., andHoffmann-Ostenhof, O. 1966. Cyclite: Biosynthese, Stoffwechsel und Vorkommen.Fortschr. Chem. Org, Nat. 24:149–205.
McLafferty, F.W. 1957. Mass spectrometric analysis.Anal. Chem. 29:1782–1789.
Miller, J.S. 1987. Phylogenetic Studies in the Papilioninae (Lepidoptera: Papilionidae).Bull. Am. Mus. Nat. Hist. 186:365–512.
Mix, D.B., Guinaudeau, H., andShamma, M. 1983. The aristolochic acids and aristolactams.J. Nat. Prod. 45:657–666.
Nishida, R. 1977. Oviposition stimulants of some Papilionid butterflies contained in their host plants.Botyu-Kagaku 42:133–140.
Nishida, R., andFukami, H. 1989. Oviposition stimulants of an Aristolochiaceae-feeding swallowtail butterfly,Atraphaneura alcinous.J. Chem. Ecol. 15:2565–2575.
Nishida, R., Ohsugi, T., Kokubo, S., andFukami, H. 1987. Oviposition stimulants of Citrusfeeding swallowtail butterfly,Papilio xuthus L.Experientia 43:342–344.
Numata, A., Hokimoto, K., Shimada, A., Yamaguchi, H., andTakaishi, K. 1979. Plant constituents biologically active to insects. I. Feeding stimulants for the larvae of the yellow butterfly.Eurema hecabe mandarina.Chem. Pharm. Bull. 27:602–608.
Numata, A., Yamaguchi, H., Hokimoto, K., Ohtani, M., andTakaishi, K. 1985. Host-plant selection by the yellow butterfly larvae,Eurema hecabe mandarina (Lepidoptera: Pieridae): Attractants and arrestants.Appl. Entomol. Zool. 20:314–321.
Ohsugi, T., Nishida, R., andFukami, H. 1985. Oviposition stimulant ofPapilio xuthus, a Citrus-feeding swallowtail butterfly.Agric. Biol. Chem. 49:1897–1900.
Ohsugi, T., Nishida, R., andFukami, H. 1991. Multi-component system of oviposition stimulants for a Rutaceae-feeding swallowtail butterfly,Papilio xuthus (Lepidoptera: Papilionidae).Appl. Entomol. Zool. 26:29–40.
Opler, P.A., andKrizek, G.O. 1984. Butterflies East of the Great Plains. Johns Hopkins University Press, Baltimore, Maryland.
Papaj, D.R. 1986a. Conditioning of leaf-shape discrimination by chemical cues in the butterfly,Battus philenor.Anim. Behav. 34:1281–1288.
Papaj, D.R. 1986b. Interpopulation differences in host-selection behavior by pipevine swallowtail butterflies (Battus philenor).Evolution 40:518–530.
Plouvier, V. 1956. Sur la recherche de quelques cyclitols: Viburnitol, scyllitol, pinitol. C.R.Acad. Sci. 242:2389–2392.
Plouvier, V. 1963. Distribution of aliphatic polyols and cyclitols, pp. 313–336,in T. Swain (ed.). Chemical Plant Taxonomy. Academic Press, London and New York.
Rausher, M.D. 1980. Host abundance, juvenile survival and oviposition preferences inBattus philenor.Evolution 34:342–355.
Reese, J.C., Chan, B.G., andWeiss, A.C., Jr. 1982. Effects of cotton condensed tannin, maysin (corn) and pinitol (soybeans) onHeliothis zea growth and development.J. Chem. Ecol. 8:1429–1436.
Renwick, J.A.A., andRadke, C.D. 1983. Chemical recognition of host plants for oviposition by the cabbage butterfly,Pieris rapae (Lepidoptera: Pieridae).Environ. Entomol. 12:446–450.
Saxena, K.N., andGoyal, S. 1978. Host-plant relations of the citrus butterflyPapilio demoleus L.: Orientational and ovipositional responses.Entomol. Exp. Appl. 24:1–10.
Saxena, K.N., andPrabha, S. 1975. Relationship between the olfactory sensilla ofPapilio demoleus L. larvae and their orientation responses to different odours.J. Entomol. Ser. A, 50:119–126.
Scriber, J.M. 1984. Larval foodplant utilization by the world Papilionidae (Lep.): Latitudinal gradients reappraised.Tokurana 6/7:1–50.
Sharon, N., andLis, H. 1989. Lectins as cell recognition molecules.Science 246:227–234.
Sherman, W.R., Eilers, N.C., andGoodwin, S.L. 1970. Combined gas chromatography-mass spectrometry of the inositol trimethylsilyl ethers and acetate esters.Org. Mass Spectrom. 3:829–840.
Silverstein, R.M., Bassler, G.C., andMorill, T.C. 1974. Spectrometric Identification of Organic Compounds. John Wiley & Sons, New York. p. 15.
Takaishi, K., Yamamoto, K., andKawahara, Y. 1969. Studies on the relationship between the food life ofLuehdorfia japonica Leech and the chemical components ofHeterotropa andAsiasarum genera.Yakugaku Zasshi 89:1144–1148.
Tarazona, J.V., andSanz, F. 1990. Toxicity of fractions obtained from the legume speciesAstragalus lusitanicus Lam.lusitanicus. Toxicon 28:235–237.
Traynier, R.M.M. 1984. Associative learning in the ovipositional behaviour of the cabbage butterfly,Pieris rapae.Physiol. Entomol. 9:465–472.
Traynier, R.M.M. 1986. Visual learning in assays of sinigrin solution as an oviposition releaser for the cabbage butterfly,Pieris rapae.Entomol. Exp. Appl. 40:25–33.
Traynier, R.M.M. 1987. Learning without neurosis in host finding and oviposition by the cabbage butterfly,Pieris rapae, pp. 243–247,in V. Labeyrie, G. Fabres, and D. Lachaise (eds.). Insects-Plants. W. Junk Publishers, Dordrecht, The Netherlands.
Tyler, H.A. 1975. The Swallowtail Butterflies of North America. Naturegraph, Healdsburg, California.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Papaj, D.R., Feeny, P., Sachdev-Gupta, K. et al. d-(+)-Pinitol, an oviposition stimulant for the pipevine swallowtail butterfly,Battus philenor . J Chem Ecol 18, 799–815 (1992). https://doi.org/10.1007/BF00994616
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00994616