Abstract
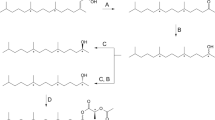
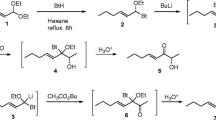
Methyl-substituted analogs of (Z)-5-decenyl acetate, a pheromone component of the turnip moth,Agrotis segetum, have been synthesized and studied by electrophysiological single-sensillum recordings and molecular mechanics calculations [MM2(85)]. The analogs are monomethyl substituted in the 2, 3, 4, and 5 positions and geminally dimethyl substituted in the 2, 3, and 4 positions. The methyl groups have been employed as space probes to study the degree of steric complementarity between the acetate-substituted alkyl chain of the pheromone component and its receptor. The electrophysiological activities, interpreted in terms of a receptor interaction model, indicate significant steric repulsive interactions between the introduced methyl groups and the receptor. This implies a high degree of complementarity between the acetate-substituted alkyl chain of the natural pheromone component and its receptor.
Similar content being viewed by others
References
Alexakis, A., Cahiez, G., andNormant, J.F. 1980. Vinyl-copper derivatives XI. Reactivity of (Z)-alkenyl cuprates towards various electrophiles. Application to the synthesis of some natural products.Tetrahedron 36:1961–1969.
Bengtsson, M., Liljefors, T., andHansson, B.S. 1987. Dienic analogues of (Z)-5-decenyl acetate, a pheromone component of the turnip moth,Agrotis segetum: Synthesis, conformational analysis and structure-activity relationships.Bioorg. Chem 15:409–422.
Bengtsson, M., Liljefors, T., Hansson, B.S., Löfstedt, C., andCopaja, S.V. 1990. Structure-activity relationships for chain-shortened analogs of (Z)-5-decenyl acetate, a pheromone component of the turnip moth,Agrotis segetum.J. Chem. Ecol. 16:667–684.
Bestmann, H.J., Rösel, P., andVostrowsky, O. 1979. Pheromones, XXV. Alkylverzweigte Analoge von Lepidopteren-pheromonen.Liebigs Ann. Chem. 1979:1189–1204.
Bestmann, H.J., Hirsch, H.L., Platz, H., Rheinwald, M., andVostrowsky, O. 1980. Differenzierung chiraler Pheromon-Analoga durch Chemorezeptoren.Angew. Chem. 92:492–493.
Burkert, U., andAllinger, N.L. 1982. Molecular Mechanics. American Chemical Society, Washington, D.C.
Chuit, C., Foulon, J.P., andNormant, J.F. 1981. Réactivité des Dérivés Organocuivreux vis- à-vis des Aldéhydes α,β-Éthyléniques.Tetrahedron 37:1385–1389.
Gardette, M., Alexakis, A., andNormant, J.F. 1985. Carbocupration of alkynes by organo copper reagents bearing a protected hydroxy or thiol function.Tetrahedron 41:5887–5899.
Hallberg, E. 1981. Fine-structural characteristics of the antennal sensilla ofAgrotis segetum (Insecta: Lepidoptera).Cell Tissue Res. 218:209–218.
Hansch, C., andLeo, A. 1979. Substituent Constants for Correlation Analysis in Chemistry and Biology. John Wiley & Sons, New York.
Houx, N.W.H., Voerman, S., andJongen, W.M.F. 1974. Purification and analysis of synthetic insect sex attractants by liquid chromatography on a silver-loaded resin.J. Chromatogr. 96:25–32.
Jonsson, S., Liljefors, T., andHansson, B.S. 1991. Alkyl substitution in terminal chain of (Z)-5-decenyl acetate, a pheromone component of turnip moth,Agrotis segetum. Synthesis, single-sensillum recordings, and structure-activity relationships.J. Chem. Ecol. 17:103–122.
Kaissling, K.-E. 1974. Sensory transduction in insect olfactory receptors, pp. 243–273,in L. Jaenicke (ed.). Biochemistry of Sensory Functions. Springer-Verlag, Berlin.
Leo, A., Hansch, C., andElkins, D. 1971. Partition coefficients and their uses.Chem. Rev. 71:525–616.
Liljefors, T. 1983. MOLBUILD-an interactive computer graphics interface to molecular mechanics.J. Mol. Graphics 1:111–117.
Liljefors, T., Thelin, B., and van derPers, J.N.C. 1984. Structure-activity relationships between stimulus molecule and response of a pheromone receptor cell in turnip moth,Agrotis segetum. Modifications of the acetate group.J. Chem. Ecol. 10:1661–1675.
Liljefors, T., Thelin, B., van derPers, J.N.C., andLöfstedt, C. 1985. Chain-elongated analogs of a pheromone component of the turnip moth,Agrotis segetum. A structure-activity study using molecular mechanics.J. Chem. Soc. Perkin Trans. 2:1957–1962.
Liuefors, T., Bengtsson, M., andHansson, B.S. 1987. Effects of double-bond configuration on interaction between a moth sex pheromone component and its receptor: A receptor-interaction model based on molecular mechanics.J. Chem. Ecol. 13:2023–2040.
Löfstedt, C., Van Der Pers, J.N.C., Löfquist, J., Lanne, B.S., Appelgren, M., Bergström, G., andThelin, B. 1982. Sex pheromone components of the turnip moth,Agrotis segetum: Chemical identification, electrophysiological evaluation and behavioral activity.J. Chem. Ecol. 8:1305–1321.
Olsson, A.-M., Jönsson, J.-Å., Thelin, B., andLiljefors, T. 1983. Determination of the vapor pressures of moth sex pheromone components by a gas Chromatographic method.J. Chem. Ecol. 9:375–385.
Pfeffer, P.E., Silbert, L.S., andChirinko, J.M. 1972. α-Anions of carboxylic acids. II. The formation and alkylation of α-metalated aliphatic acids.J. Org. Chem. 37:451–458.
Priesner, E. 1979. Specificity studies on pheromone receptors in noctuid and tortricid Lepidoptera, pp. 57–71,in F.J. Ritter (ed.). Chemical Ecology: Odour Communications in Animals. Elsevier/North Holland, Amsterdam.
Priesner, E., Bestmann, H.J., Vostrowsky, O., andRösel, P. 1977. Sensory efficacy of alkylbranched pheromone analogues in noctuid and tortricid Lepidoptera.Z. Naturforsch. 32c:979–991.
Schwarz, M., Klun, J.A., Fritz, G.L., Uebel, E.C., andRaina, A.K. 1989. European corn borer sex pheromone: Structure-activity relationships.J. Chem. Ecol. 15:601–617.
StÄllberg-Stenhagen, S. 1946. Optically active higher aliphatic compounds. I. The synthesis of (+)-2-methyldodecanoic and (+)-2-methylhexacosanoic acids.Arkiv Kemi, Min., Geol. 23A(15):1–14.
van der Pers, J.N.C., andDen Otter, C.J. 1978. Single cell responses from olfactory receptor of small ermine moths (Lepidoptera: Yponomeutidae) to sex attractants.J. Insect Physiol. 24:337–343.
van der Pers, J.N.C., andLöfstedt, C. 1983. Continuous single sensillum recording as a detection method for moth pheromone components in the effluent of a gas Chromatograph.Physiol. Entomol. 8:203–211.
von der Lieth, C.W., Carter, R.E., Dolata, D.P., andLiuefors, T. 1984. RINGS-a general program to build ring systems.J. Mol. Graphics 2:117–123.
Weast, R.C. (ed.). 1971. Handbook of Chemistry and Physics, 52nd ed. Chemical Rubber Publishing Company, Cleveland, Ohio.
Author information
Authors and Affiliations
Additional information
Schiff., Lepidoptera: Noctuidae.
Rights and permissions
About this article
Cite this article
Jönsson, S., Liljefors, T. & Hansson, B.S. Introduction of methyl groups to acetate substituted chain of (Z)-5-decenyl acetate, a pheromone component of turnip moth,agrotis segetum: synthesis, single-sensillum recordings, and structure-activity relationships. J Chem Ecol 18, 637–657 (1992). https://doi.org/10.1007/BF00987825
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00987825