Abstract
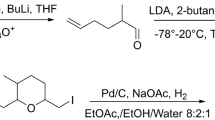
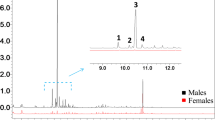
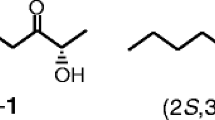
The enantiomeric composition of the pheromone components (+)-ipsdienoI, e.e. 87.6%, and (−)-ipsenol, e.e. 93.8%, produced by the male bark beetleIps paraconfusus (Scolytidae) under natural conditions was determined by HPLC separation of their diastereomeric ester derivatives. Males confined in an atmosphere of ipsdienone produced (−)-ipsdienol, e.e. 28%, and (−)-ipsenol, e.e. 86%, indicating an enantiomeric selectivity in the conversion of the ketone to the alcohols. These findings demonstrate an enantioselective conversion mechanism in the biosynthetic pathway to the pheromones from myrcene, a host-plant terpene.
Similar content being viewed by others
References
Bergot, B.J., Anderson, R.J., Schooley, D.A., andHenrick, C.A. 1978. Liquid chromatographic analysis of enantiomeric purity of several terpenoid acids as their 1-(1-naphthyl) ethylamide derivatives.J. Chromatogr. 155:97–105.
Birch, M.C., Light, D.M., Wood, D.L., Browne, L.E., Silverstein, R.M., Bergot, B.J., Ohloff, G., West, J.R., andYoung, J.C. 1980. Pheromone attraction and allomonal interruption ofIps pini in California by the two enantiomers of ipsdienol.J. Chem. Ecol. 6:703–717.
Browne, L.E., Birch, M.C., andWood, D.L. 1974. Novel trapping and delivery systems for airborne insect pheromones.J. Insect Physiol. 20:183–193.
Byers, J.A. 1981. Pheromone biosynthesis in the bark beetle,Ips paraconfusus, during feeding or exposure to vapors of host plant precursors.Insect Biochem. 11:563–570.
Byers, J.A., Wood, D.L., Browne, L.E., Fish, R.H., Piatek, B., andHendry, L.B. 1979. Relationship between a host plant compound, mrycene and pheromone production in the bark beetle,Ips paraconfusus J. Insect Physiol. 25:477–482.
Fish, R.H., Browne, L.E., Wood, D.L., andHendry, L.B. 1979. Pheromone biosynthetic pathways: Conversions of deuterium labelled ipsdienol with sexual and enantioselectivity inIps paraconfusus Lanier.Tetrahedron Lett. 17:1465–1468.
Hendry, L.B., Piatek, B., Browne, L.E., Wood, D.L., Byers, J.A., Fish, R.H., andHicks, R.A. 1980.In vivo conversion of a labelled host plant chemical to pheromones of the bark beetleIps paraconfusus.Nature 284:485.
Hughes, P.R. 1974. Myrcene: A precursor of pheromones inIps beetles.J. Insect Physiol. 20:1271–1275.
Hughes, P.R., andRenwick, J.A.A. 1977. Neural and hormonal control of pheromone biosynthesis in the bark beetle,Ips. paraconfusus.Physiol Entomol. 2:117–123.
Mori, K. 1979. Synthesis of optically active forms of ipsdienol and ipsenol.Tetrahedron 35:933–940.
Plummer, E.L., Stewart, T.E., Byrne, K., Pearce, G.T., andSilverstein, R.M. 1976. Determination of enantiomeric composition of several insect pheromone alcohols.J. Chem. Ecol. 2:307–331.
Silverstein, R.M., Rodin, J.O., andWood, D.L. 1966a. Sex attractants in the frass produced by maleIps confusus in ponderosa pine.Science 154:509–510.
Silverstein, R.M., Rodin, J.O., Wood, D.L., andBrowne, L.E. 1966b. Identification of two new terpene alcohols from frass produced byIps confusus in ponderosa pine.Tetrahedron 22:1929–1936.
Wood, D.L., Browne, L.E., Silverstein, R.M., andRodin, J.O. 1966. Sex pheromones of bark beetles. I. mass production, bioassay, and isolation of the sex pheromone ofIps confusus (LeC.).J. Insect Physiol. 12:523–536.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fish, R.H., Browne, L.E. & Bergot, B.J. Pheromone biosynthetic pathways: Conversion of ipsdienone to (−)-ipsdienol, a mechanism for enantioselective reduction in the male bark beetle,Ips paraconfusus . J Chem Ecol 10, 1057–1064 (1984). https://doi.org/10.1007/BF00987512
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00987512