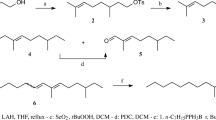
Abstract
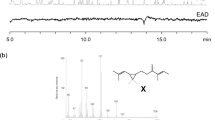
Gas chromatographic-electroantennographic analysis (GC-EAD) of female larch looper,Semiothisa sexmaculata (Packard), gland extracts revealed two EAD-active compounds. Retention index calculations, GC-mass spectroscopy in selected ion monitoring mode, and GC-EAD analysis of authentic standards identified the compounds as (3Z,6Z,9Z)-heptadecatriene (3Z,6Z,9Z-17∶H) and (6Z,9Z)-cis-3,4-epoxy-heptadecadiene (6Z,9Z-cis-3,4-epoxy-17∶H). Chirality determination of the monoepoxydiene in gland extracts was impeded by small quantities, but field experiments indicated that maleS. sexmaculata were most strongly attracted to enantiomerically enriched 6Z,9Z-3R,4S-epoxy-17∶H (69% ee), while maleS. neptaria (Guenée) responded well to various blends of theR,S- and S,R-epoxide enantiomers. Binary combinations of theR,S-epoxide enantiomer with 3Z,6Z,9Z-17∶H significantly inhibited response by maleS. sexmaculata, but strongly enhanced attraction of sympatric maleS. marmorata Ferguson. Enantiomerically enriched 6Z,9Z-3R,4S-epoxy-17∶H can be used as a trap bait to monitor populations of the larch-defoliatingS. sexmaculata. Whether 6Z,9Z-3R,4S-epoxy-17∶H serves as single component sex pheromone inS. sexmaculata or small amounts of 6Z,9Z-3S,4R-epoxy-17∶H synergize or suppress optimal attraction, will be tested as chirally pure monoepoxydienes become available.
Similar content being viewed by others
References
Arn, H., Städler, E., andRauscher, S. 1975. The electroantennographic detector-a selective and sensitive tool in the gas chromatographic analysis of insect pheromones.Z. Naturforsch. 30c:722–725.
Furniss, A.L., andCarolin, V.M. 1978. Western forest insects. US Department of Agriculture Forest Service Miscellaneous Publication No. 1339, pp. 654.
Hansson, B.S., Szöcs, G., Schmidt, F., Francke, W., Löfstedt, C., Tóth, M. 1990. Electrophysiological and chemical analysis of the sex pheromone communication system of the mottled umber,Erannis defoliaria (Lepidoptera: Geometridae).J. Chem. Ecol. 16:1887–1897.
Millar, J.G., Underhill, E.W., Giblin, M., andBarton, D. 1987. Sex pheromone components of three species ofSemiothisa (Geometridae), (Z,Z,Z)-3,6,9-heptadecatriene and two monoepoxydiene analogs.J. Chem. Ecol. 13:1371–1383.
Millar, J.G., Giblin, M., Barton, D., andUnderhill, E.W. 1990a. (3Z,6Z,9Z)-Nonadecatriene and enantiomers of (3Z,9Z)-cis-6,7-epoxy-nonadecadiene as sex attractants for two geometrid and one noctuid moth species.J. Chem. Ecol. 16:2153–2166.
Millar, J.G., Giblin, M., Barton, D., andUnderhill, E.W. 1990b. 3Z,6Z,9Z-Trienes and unsaturated epoxides as sex attractants for geometrid moths.J. Chem. Ecol. 1:2307–2316.
Millar, J.G., Giblin, M., Barton, D., Morrison, A., andUnderhill, E.W. 1990c. Synthesis and field testing of enantiomers of 6Z,9Z-cis-3,4-epoxydienes as sex attractants for geometrid moths. Interaction of enantiomers and regioisomers.J. Chem. Ecol. 17:2317–2339.
Millar, J.G., Giblin, M., Barton, D., andUnderhill, E.W. 1991. Chiral lepidopteran sex attractants: Blends of optically active C20 and C21 diene epoxides as sex attractants for geometrid and noctuid moths (Lepidoptera).Environ. Entomol. 20:450–457.
Underhill, E.W., Palaniswamy, P., Abrams, S.R., Bailey, B.K., Steck, W.F., andChisholm, M.D. 1983. Triunsaturated hydrocarbons, sex pheromone components ofCaenurgina erected.J. Chem. Ecol. 9:1413–1423.
Underhill, E.W.,Millar, J.G., andWong, J.W. 1985. Unsaturated hydrocarbons and their oxygenated analogs as a major class of lepidopteran sex attractants. Entomological Society of America National Meeting., December 1985, Hollywood, Florida.
Unger, L., andVallentgoed, J. 1990. Forest insect and disease conditions. Nelson Forest Region, Forestry Canada,FIDS Report 91-3: pp. 1–51.
Wong, J.W., Underhill, E.W., Mackenzie, S.L., andChisholm, M.D. 1985. Sex attractants for geometrid and noctuid moths. Field trapping and electroantennographic responses to triene hydrocarbons and monoepoxydiene derivatives.J. Chem. Ecol. 11:727–756.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gries, G., Gries, R., Underhill, E.W. et al. (6Z,9Z-3R,4S)-Epoxy-heptadecadiene: major sex pheromone component of the larch looper,Semiothisa sexmaculata (Packard) (Lepidoptera: Geometridae). J Chem Ecol 19, 843–850 (1993). https://doi.org/10.1007/BF00985014
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00985014