Abstract
Eleven analogs of (E,Z)-2,13-octadecadien-1-yl acetate1, a main pheromone component of the currant clearwing moth,Synanthedon tipuliformis Clerk (Lepidoptera: Sesiidae) were synthesized and tested for their biological activities by electroantennography (EAG). To correct the EAG data for differences in volatility of the analogs, their vapor pressures were estimated by a gas chromatographic method. All structural changes in the parent molecule were found to reduce the biological activity to various degrees. The most active analog tested was the carbamate12, whose activity was almost comparable to that of the pheromone component1. Structure-activity correlations showed that hydrophobic, steric, and electronic effects of chain terminal groups might be responsible for variations in biological activity of the conformationally unchanged (E,Z)-2,13-analogs
Similar content being viewed by others
References
Albans, K.R., Baker, R., Jones, O.T., andTurnbull, M.D. 1984. Inhibition of response ofHeliothis virescens to its natural pheromone by antipheromones.Crop. Prot. 3:501–506.
Arn, H.,Tóth, M., andPriesner, E. 1986. List of Sex Pheromones of Lepidoptera and Related Attractants, OILB-SROP, Paris, 123 pp.
Attenburrow, J., Cameron, A.F.B., Chapman, J.H., Evans, R.M., Hems, B.A., Jansen, A.B.A., andWalker, T. 1952. A synthesis of vitamin A from cyclohexanone.J. Chem. Soc. 1952:1094–1111.
Bengtsson, M., Liljefors, T., andHansson, B.S. 1987. Dienic analogs of (Z)-5-decenyl acetate, a pheromone component of the turnip moth,Agrotis segetum: Synthesis, conformational analysis and structure-activity relationships.Bioorg. Chem. 15:409–422.
Bengtsson, M., Liljefors, T., Hansson, B.S., Löfstedt, C., andCopaja, S.V. 1990. Structureactivity relationships for chain-shortened analogs of (Z)-5-decenyl acetate, a pheromone component of the turnip moth.Agrotis segetum.J. Chem. Ecol. 16:667–684.
Bestmann, H.J., Cai-Hong, W., Döhla, B., Li-Kedono, andKaissling, K.E. 1987. Functional group recognition of pheromone molecules by sensory cells ofAntheraea polyphemus andAntheraea pernyi (Lepidoptera: Saturnidae).Z. Naturforsch. 42c:435–441.
Bidleman, T.F. 1984. Estimation of vapor pressures for nonpolar organic compounds by capillary gas chromatography.Anal. Chem. 56:2490–2496.
Brown, C.A., andAhuja, K.K. 1973. “P2-nickel” catalyst with ethylendiamine, a novel system for highly stereospecific reduction of alkynes tocis-olefins.J. Chem. Soc. Chem. Commun. 1973:553–561.
Camps, F., Fabri, S.G., Gasol, V., Guerrero, A., Hernández, R., andMontoya, R. 1988. Analogs of sex pheromone of processionary moth,Thaumetopoea pityocampa: Synthesis and biological activity.J. Chem. Ecol. 14:1331–1346.
Evershed, R.P. 1988. Insect olfaction and molecular structure, pp. 1–33,in E.D. Morgan and N.B. Mandava (eds.). CRC Handbook of Natural Pesticides. Vol IV., Pheromones, Part A. CRC Press, Boca Raton, Florida.
Hamilton, D.J. 1980. Gas chromatographics measurement of volatility of herbicide esters.J. Chromatogr. 195:75–83.
Hansch, C., andLeo, A. 1979. Substituents Constants for Correlation Analysis in Chemistry and Biology. John Wiley & Sons, New York.
Hopfinoer, A.J. 1980. A QSAR investigation of dihydrofolate reductase inhibition by Baker triazines based on molecular shape analysis.J. Am. Chem. Soc. 102:7196–7206.
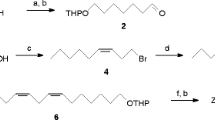
Hoskovec, M., Šaman, D., andKoutek, B. 1990. A convenient synthesis of 2,13- and 3,13-octadecadienyl acetates, sex pheromone components of theSynanthedon species.Collect. Czech. Chem. Commun. 55:2270–2281.
Jönsson, S., Liljefors, T., andHansson, B.S. 1991a. Alkyl substitution in terminal chain of (Z)-5-decenyl acetate, a pheromone component of turnip moth,Agrotis segetum. Synthesis, single-sensillum recordings, and structure-activity relationships.J. Chem. Ecol. 17:103–122.
Jönsson, S., Liljefors, T., andHansson, B.S. 1991b. Replacement of the terminal methyl group in a moth sex pheromone component by a halogen atom: Hydrophobicity and size effects on electrophysiological single-cell activities.J. Chem. Ecol. 17:1381–1397.
Koutek, B., Hoskovec, M., Konečný, K., andVrkoč, J., 1992. Gas chromatographic determination of vapor pressures of pheromone-like acetates.J. Chromatogr. 626:215–221.
Liljefors, T., Thelin, B., andVan der Pers, J.N.C. 1984. Structure-activity relationships between stimulus molecule and response of a pheromone receptor cell in the turnip moth,Agrotis segetum. Modifications of the acetate group.J. Chem. Ecol. 10:1661–1675.
Liljefors, T., Thelin, B., Van der Pers, J.N.C., andLössted, C. 1985. of the turnip moth,Agrotis segetum. A structure-activity study using molecular mechanics.J. Chem. Soc. Perkin Trans. 2:1957–1962.
Liljefors, T., Bengtsson, M., andHansson, B.S. 1987. Effects of double-bond configuration on interaction between a moth sex pheromone component and its receptor. A receptor-interaction model based on molecular mechanics.J. Chem. Ecol. 13:2023–2040.
Mackay, D., Bobra, A., Chan, D.W., andShiu, W.Y. 1982. Vapor pressure correlations for low volatility environmental chemicals.Environ. Sci. Technol. 16:645–649.
Macknick, A.B., andPrausnitz, J.M. 1979. Vapor pressures of high-molecular-weight hydro-carbons.J. Chem. Eng. Data 24:175–178.
Prestwich, G.D. 1987. Chemical studies of pheromone reception and catabolism, pp. 473–528,in G.D. Prestwich and G.J. Blomquist (eds.). Pheromone Biochemistry. Academic Press, Orlando, Florida.
Ramiandrasoa, F., andDescoins, C. 1989. A new highly stereospecific synthesis of (E,Z)-2,13-octadecadienyl acetate, a sex pheromone component of Lepidoptera species.Synth. Commun. 19:2703–2712.
Sharma, M.L., Thapar, S., andGupta, R. 1990. Synthesis of (2E,13Z)-2,13-octadecadien-1-yl acetate.Ind. J. Chem. 29b:657–658.
Sorochinskaya, A.M., andKovalev, B.G. 1991. Synthesis oftrans-2,cis-13 and trans-3,cis- 13-octadecadien-1-yl acetates, pheromone component of theSynanthedon tipuliformis (Lepidoptera: Sesiidae). (in Russian)Zh. Org. Khim. 27:722–727.
Szöcs, G., Miller, L.A., Thomas, W., Vickers, R.A., Rotschild, G.H.L., Schwarz, M., andTóth, M. 1990. Compounds modifying male responsiveness to main female sex currant borer,Synanthedon tipuliformis Clerk (Lepidoptera: Sesiidae) under field conditions.J. Chem. Ecol. 16:1289–1305.
Szöcs, G., Buda, V., Charmillot, P., Esbjerg, P., Freier, B., Gottwald, R., Kovalev, B., Maini, S., Solomon, M.G., Sorum, O., Subchev, M., Tóth, M., andVan Der Veire, M. 1991. Field tests of (E,Z)-3,13-octadecadien-1-ol acetate: A sex attractant Synergist for male currant borer,Synanthedon tipuliformis.Entomol. Exp. Appl. 60:283–288.
Tonini, C., Cassani, G., Massardo, P., Guglielmetti, G., andCastellari, P.L. 1986. Study of female sex pheromone of leopard moth,Zeuzera pyrina L. Isolation and identification of three component.J. Chem. Ecol. 12:1545–1558.
Verloop, A., Hoogenstraaten, W., andTipker, J. 1976. Development and application of new steric substituent parameters in drug design, pp. 165–207in E.J. Ariens (ed.). Drug Design, Vol. VII. Academic Press, New York.
Voerman, S., andRothschild, G.H.L. 1978. Synthesis of the two components of the sex pheromone system of the potato tubeworm moth,Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae) and field experience with them.J. Chem. Ecol. 4:531–542.
Voerman, S., Persoons, C.J., andPriesner, E. 1984. Sex attractant for currant clearwing mothSynanthedon tipuliformis Clerk (Lepidoptera, Sesiidae).J. Chem. Ecol. 10:1371–1375.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hoskovec, M., Kalinová, B., Konečný, K. et al. Structure-activity correlations among analogs of the currant clearwing moth pheromone. J Chem Ecol 19, 735–750 (1993). https://doi.org/10.1007/BF00985005
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00985005