Abstract
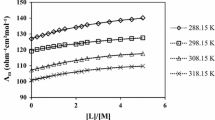
The kinetics of the solvolysis of [Co(CN)5Cl]3− have been investigated in water +2-methoxyethanol and water + diethylene glycol mixtures. Although the addition of these linear hydrophilic cosolvent molecules to water produces curvature in the variation of log(rate constant) with the reciprocal of the dielectric constant, their effect on the enthalpy and entropy of activation is minimal, unlike the effect of hydrophobic cosolvents. The application of a Gibbs energy cycle to the solvolysis in water and in the mixtures using either solvent-sorting or TATB values for the Gibbs energy of transfer of the chloride ion between water and the mixture shows that the relative stability of the emergent solvated Co(III) ion in the transition state compared to that of Co(CN)5Cl3− in the initial state increases with increasing content of cosolvent in the mixture. By comparing the effects of other cosolvents on the solvolysis, this differential increase in the relative stabilities of the two species increases with the degree of hydrophobicity of the cosolvent.
Similar content being viewed by others
Abbreviations
- v2 :
-
partial molar volume of the cosolvent in water + cosolvent mixtures
- V o2 :
-
molar volume of the pure cosolvent
- ΔH Emix :
-
excess enthalpy of mixing water and cosolvent
- ΔS Emix :
-
excess entropy of mixing water and cosolvent
- ΔG ot (i)n :
-
the Gibbs energy of transfer of speciesi from water into the water + cosolvent mixture excluding electrostatic contributions
- k s :
-
first order rate constant for the solvolysis in water + cosolvent mixtures
- D s :
-
dielectric constant of the water + cosolvent mixture
- ΔH * :
-
the enthalpy of activation for the solvolysis
- ΔS * :
-
the entropy of activation for the solvolysis
- ΔG * :
-
the Gibbs energy of activation for the solvolysis
- ΔV * :
-
the volume of activation for the solvolysis
- i * :
-
speciesi in the transition state for the solvolysis
- H o :
-
Hammett Acidity Function
- TATB:
-
method for estimating the Gibbs energy of transfer for single ions assuming those for Ph4As+ and BPh −4 are equal
References
K. H. Halawani and C. F. Wells,J. Chem. Soc. Faraday Trans. 86, 1791 (1990).
K. H. Halawani and C. F. Wells,Trans. Metal Chem.,18, 149 (1993).
G. M. El-Subruiti, I. M. Sidahmed and C. F. Wells,J. Solution Chem.,21, 93 (1992).
K. H. Halawani and C. F. Wells,J. Solution Chem.,19, 1073 (1990).
G. Wada and S. Umeda,Bull. Chem. Soc. Japan,35, 646 (1962).
K. Nakanish,Bull. Chem. Soc. Japan,33, 793 (1960).
J. Kenttämaa, E. Tommila and M. Martti,Ann. Acad. Scient. Fenn., A,No. 93 (1959).
R. F. Lama and B. C.-Y. Lu,J. Chem. Eng. Data,10, 216 (1965).
J. H. Andreae, P. D. Edmonds and J. F. McKellar,Acustica,15, 74 (1965).
K. J. Laidler and P. A. Landskroener,Trans. Faraday Soc,52, 200 (1956).
C. F. Wells,J. Chem. Soc., Faraday Trans. I,73, 1851 (1977).
K. H. Halawani and C. F. Wells,Int. J. Chem. Kin.,24, 1043 (1992).
A. Ray and G. Némethy,J. Chem. Eng. Data,18, 309 (1973).
K. Rehm and H.-J. Bittrich,Z. phys Chem. Leipzig,251, 109 (1972).
M. A. Villamanan, C. Gonzalez and H. C. Van Ness,J. Chem. Eng. Data,29, 427 (1984).
J. B. Taylor and J. S. Rowlinson,Trans. Faraday Soc.,51, 1183 (1955).
K. H. Hawalani and C. F. Wells,J. Chem. Soc. Faraday Trans,89, 3565 (1993).
G. Wada and S. Umeda,Bull. Chem. Soc. Japan,35, 1797 (1962).
H. Nakayama and K Shinoda,J. Chem Thermodynamics,3, 401 (1971).
K. H. Halawani and C. F. Wells,Thermochim. Acta,191, 121 (1991).
W. J. Wallace and A. L. Mathews,J. Chem. Eng. Data,8, 496 (1963).
J. Saxton and C. F. Wells,J. Chem. Soc. Faraday Trans.,86, 1471 (1990).
C. Kalidas and V. Srinivas Rao,J. Chem. Eng. Data,19, 201 (1974).
H. Sadek, Th. F. Tadros and A. A. El-Harakany,Electrochim. Acta,16, 339 (1971).
G. Douhéret and A. Pal,J. Chem. Eng. Data,33, 40 (1988).
G. M. El-Subruiti, I. M. Sidahmed and C. F. Wells,Int. J. Chem. Kin. 24, 563 (1992).
D. A. Palmer and H. Kelm,Z. anorg. allg. Chem.,450, 50 (1979).
M. G. Burnett and M. W. Gilfillan,J. Chem. Soc. Dalton Trans., 1578 (1981); M. H. M. Abu-El-Wafa and M. G. Burnett,J. Chem. Soc. Chem. Comm., 833 (1983); M. H. M. Abou-El-Wafa, M. G. Burnett and J. F. McCullagh,J. Chem. Soc. Dalton Trans., 1059, 2311 (1987).
K. H. Halawani and C. F. Wells,J. Chem. Soc. Faraday Trans. I,85, 2185 (1989).
C. F. Wells,Australian J. Chem.,36, 1739 (1983).
C. F. Wells,Thermochim. Acta,185, 183 (1991).
A. Battacharya, A. K. Das and K. K. Kundu,Indian J. Chem.,20A, 347 (1981).
C. F. Wells,Thermochim. Acta,200, 443 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Halawani, K.H., Wells, C.F. The kinetics of the solvolysis of chloropentacyanocobaltate(III) ions in water containing 2-methoxyethanol or diethylene glycol: A contrast with the effect of more hydrophobic cosolvents. J Solution Chem 23, 1089–1100 (1994). https://doi.org/10.1007/BF00976258
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00976258