Abstract
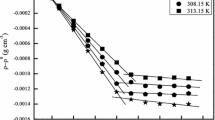
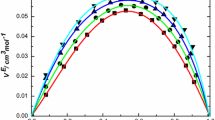
Density and viscosity of binary systems water-nonionic surfactants poly(ethylen-oxide) alkyl alcohols type, [CnH2n+1(OCH2CH2)mOH, CnEm], have been studied. The partial molar volumes in the dilute solution range and the viscosity B-coefficients were calculated. The nonionic surfactants partial molar volumes were compared with those of ethylene glycol and poly(ethylenglycol) (PEG). The comparison shows that the ethoxy unit volume,\(\bar V^O \) (OCH2CH2), seems to be independent of the particular system. The consequences of this are discussed. A model for interpreting the experimental B values has been proposed. The model treats the macroscopic viscosity as the superimposition of different local effects. The following surfactants have been considered: C5E1, C5E2, C6E1, C6E2, C6E3, C6E4.
Similar content being viewed by others
References
J. Traube,Samml. Chem. Vortr. 4, 255 (1899).
S. Cabani, C. Conti, and L. Lepori,J. Phys. Chem. 78, 1030 (1974).
L. Lepori and V. Mollica,J. Chem. Eng. Dats 23, 65 (1978).
L. Ambrosone, R. Sartorio, A. Vescio, and V. Vitagliano,J. Chem. Soc. Faraday Trans. 92, 1163 (1996).
H. Wennerstrom and B. Lindman,Physics Reports (Review Section of Physics Letters)52, 1 (1979).
Nonionic surfactants. Physical chemistry. ed. Martin J. Schick (Author, publisher, place & date)
G. Douhéret, A. Pal, and M. I. Davis,J. Chem. Thermodyn. 22, 99 (1990).
G. Douhéret, C. Salgado, M. I. Davis, and J. Loya,Thermochimica Acta 207, 313 (1992).
G. Roux, G. Perron, and J. E. Desnoyers,J. Solution Chem. 7, 639 (1978).
S. A. Wieczorek,J. Chem. Thermodyn. 24, 129 (1992).
H. S. Frank and M. W. Evans,J. Chem. Phys. 13, 507 (1945).
W. Kauzmann,Ad. Protein Chem. 14, 1 (1959).
W. Blokzijl and B. F. N. Engberts,Angew. Chem. Int. Ed. Engl. 32, 1545 (1993).
G. S. Kell,J. Chem. Eng. Dats 20, 97 (1975).
A. Lo Surdo, E. M. Alzola, and F. J. Millero,J. Chem. Thermodynamics 14, 649 (1982).
Handbook of Chemistry and Physics, CRC Press, Boca Raton, FL, 59th edn. (1978).
L. Lepori and V. Mollica,J. Polymer Sci. Polym. Phys. Ed. 16, 1123 (1978).
S. Terasawa, H. Itsuki, and S. Arakawa,J. Phys. Chem. 22, 2345 (1975).
J. T. Edward, P. G. Farrell, and F. Shahidi,J. C. S. Faraday Trans. 73, 705 (1977).
J. T. Edward, P. G. Farrell, and F. Shahidi,J. C. S. Faraday Trans. 73, 715 (1977).
H. Hoøiland and E. Vikingstad,Acta Chem. Scand, Ser. A. 30, 182 (1976).
H. S. Wu and S. I. Sandler,Ind. Eng. Chem. Res. 30, 881 (1991).
H. S. Wu and S. I. Sandler,Ind. Eng. Chem. Res. 30, 889 (1991).
S. Wurzburger, R. Sartorio, V. Elia, and C. Cascella,J. Chem. Soc. Faraday Trans. 86, 3891 (1990).
D. M. Alexander and D. C. Moy,Aust. J. Chem. 35, 465 (1982).
T. T. Herskovits and T. M. Kelly,J. Phys. Chem. 77, 381 (1973).
H. L. Frish and R. Simha, inRheology, Vol. I. ed. R. Eirick, (Academic Press inc, New York 1956), p. 525.
R. V. Gurney,Ionic Process in Solution, (McGraw-Hill, London 1953).
L. Paduano, R. Sartorio, V. Vitagliano, and L. Costantino, to be published.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ambrosone, L., Costantino, L., D'Errico, G. et al. Density and viscosity studies of poly(ethylene-oxide) alkyl alcohols. J Solution Chem 25, 757–772 (1996). https://doi.org/10.1007/BF00973783
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00973783